NPF COVID-19 Task Force: Masks, Social Distancing Still Important for Many Vaccinated PsO Patients
The NPF COVID-19 Task Force has updated its guidance statements in response to the CDC’s new public health recommendations for fully vaccinated individuals, released last month. The CDC guidance states that fully vaccinated people no longer need to wear a mask or physically distance in any setting, except where required by law, business policy, or workplace guidance, but NPF is providing additional clarity for people living with psoriatic disease.
Since patients with psoriasis and/or psoriatic arthritis may be taking medications that affect the immune system in a manner that may increase the risk of infections, they may not be fully protected even if fully vaccinated against COVID-19. NPF recommends that patients with psoriatic disease taking abatacept, cyclosporine, leflunomide, glucocorticoids (e.g., prednisone), methotrexate, or tofacitinib continue masking and social distancing precautions when they are in contact with people not vaccinated against COVID-19 or whose vaccination status is not verifiable.
The NPF Task Force also advises against antibody testing to assess immunity after COVID-19 vaccination or to inform medical decision making related to individual precautions. Currently, the accuracy of antibody testing to predict protection from SARS-CoV-2 infection and COVID-19 illness is not known.
“The NPF COVID-19 Task Force is excited about the release of the new CDC guidelines,” says Joel M. Gelfand, MD, MSCE, Professor of Dermatology and Epidemiology at the University of Pennsylvania Perelman School of Medicine and Co-Chair of the NPF COVID-19 Task Force, in a news release. “It’s important to emphasize that the COVID-19 vaccines are highly effective, especially by dramatically lowering the risk of COVID-19 hospitalization and death, as well as reducing the ability of a vaccinated person to spread the virus. While emerging data suggest that some more broadly immune targeted treatments, such as methotrexate, may slightly reduce the antibody response to COVID-19 vaccines, patients on these treatments should still get vaccinated as they likely still provide protection from severe COVID-19 illness. Our recommendations are out of an abundance of caution until we have more data. While we await more research, the best way to prevent COVID-19 is to get vaccinated and if there is reason to be concerned that you might not have an optimal vaccine response due to the medications you are taking for psoriatic disease, then continue to mask and distance when you are in contact with people who are not vaccinated against COVID-19.”
AADA Issues Statement on Legislative Interference in Health Care for Transgender Patients
The American Academy of Dermatology Association (AADA) says legislation, such as the bill enacted this spring in Arkansas regarding transgender patients, are a dangerous intrusion by government into medical decision-making. In response to legislation proposed in several states, AADA President Kenneth J. Tomecki, MD, FAAD issued the following statement:
The American Academy of Dermatology Association strongly opposes recent efforts by state legislatures to restrict physicians’ ability to provide care to transgender youths. Legislation such as the bill enacted this spring in Arkansas as well as those proposed in several other states are a dangerous intrusion by government into medical decision-making.
The AADA recognizes the dignity and identity of transgender individuals and advocates for dermatologists’ ability to provide therapy and procedures that help the mental and physical well-being of these and all patients. Evidence has shown that transgender individuals who are forced to forgo gender-affirming care face an increased risk of mental health disorders including substance abuse disorders, and have higher rates of suicide.
Transgender and gender-diverse individuals can benefit greatly from medical and surgical gender-affirming treatments. These treatments are often medically necessary for the health and well-being of these patients and are not to be considered as cosmetic or elective.
Decisions about care should remain within the confines of the physician-patient relationship, guided by strong medical evidence and the best interests of the individual patient.
Cosentyx Now Approved for Psoriasis in Children as Young as Six

The FDA has approved Cosentyx (secukinumab) from Novartis for the treatment of moderate to severe plaque psoriasis in pediatric patients six years and older who are candidates for systemic therapy or phototherapy.
Cosentyx is approved for dosing of 75mg or 150mg, depending on the child’s weight, every four weeks after an initial loading regimen. After initial counseling and proper training in injection technique, Cosentyx can be administered by an adult caregiver outside of a health care provider’s office via a single-dose prefilled syringe or Sensoready pen.
This approval is based on two Phase 3 studies evaluating the use of Cosentyx in children aged 6 to <18 years with plaque psoriasis. The safety profile reported in these trials was consistent with the safety profile reported in adult plaque psoriasis trials. No new safety signals were observed.
In a 52-week (236 weeks total), randomized, double-blind, placebo- and active-controlled study that included 162 children six years of age and older with severe plaque psoriasis, Cosentyx reduced psoriasis severity at week 12 compared with placebo. A second Phase 3 study was a randomized open-label, 208-week trial of 84 subjects six years of age and older with moderate to severe plaque psoriasis.
“Treating moderate to severe plaque psoriasis in children can be complicated, as we need to balance the ability of a treatment to provide symptom relief while considering the safety profile as the top priority,” says John Browning, MD, FAAD, FAAP, MBA, clinical trial investigator, Adjunct Associate Professor of Pediatrics and Dermatology at the University of Texas Health, San Antonio. “In the pediatric pivotal study, the majority of patients treated with Cosentyx were able to achieve clear or almost clear skin with a safety profile consistent with previous clinical trials in adults. Due to the systemic nature of the disease, Cosentyx is a welcome addition as a treatment option for families dealing with this challenging condition.”
“Living with psoriasis is challenging, and can be highly stressful for children and adolescents,” says Randy Beranek, President and CEO, National Psoriasis Foundation. “Having expanded treatment options for this patient population is a step in the right direction to help reduce the burden of plaque psoriasis.”
Johnson & Johnson Consumer Health Commits to Science-Based Innovations for Skin Health
At this year’s American Academy of Dermatology (AAD) Virtual Meeting Experience (VMX), Johnson & Johnson Consumer Health presented new research including the first of its kind study on restorative skincare for cancer patients, advances in multicultural skincare, and more. The company also shared its ongoing commitment to increasing diversity in clinical trials, driving new culturally competent care solutions, and expanding innovations in skin health options for all.
“At Johnson & Johnson Consumer Health, we pride ourselves on developing innovative solutions through next-generation science for healthier skin,” said Caroline Tillett, Global Head R&D, Johnson & Johnson Consumer Health. “Over the past year, we made substantial advancements in critical research programs that will support major consumer health needs. Our new research with cancer patients, acne sufferers, and diverse communities demonstrates our unwavering commitment to provide skincare options across the spectrum of skin types, tones, and needs.”
Menas Kizoulis, Scientific Engagement Director, says the company has long been committed to research that includes multiculturally diverse subjects with skin of all colors. He adds that the company has also recognized the need to highlight the insights learned and make the research results about different skin types and tones more easily accessible. He points to research showing the effectiveness of 1% Colloidal Oatmeal OTC Cream for managing mild to moderate atopic dermatitis (AD) in African American children, a population who has a slightly higher incidence of AD.
Johnson & Johnson Consumer Health has also unveiled its new Invisible Daily Defense sunscree. Sun protection and sunscreen is essential for everyone, including patients with skin of color. Despite misconceptions that Black and Brown communities do not need sunscreen, everyone needs to protect their skin to prevent photodamage and skin cancer.
“Sun protection and sunburn can be experienced across all skin types” says Kizoulis, adding that there are known barriers to sunscreen use, particularly among people with darker skin types, including that sunscreen remains visible on their skin. Invisible Daily Defense offers a formulation innovation that can be used on all skin tones.
Education about the need for sunscreen is also a major barrier to use. Kizoulis explains that it’s 10 times less likely for a doctor to counsel a Black patient about sunscreen use. Neutrogena supported a webinar, Suncare for All: New Insights and Solutions for Protecting Patients of All Skin Tones, with ODAC to share insights about the importance of sun protection across all ethnicities, including effective counseling strategies to encourage sun safe behaviors among all patients. They also shared In the Sun documentary, executive produced by award-winning actress Kerry Washington. Patients can view the documentary for free at InTheSunFilm.com. In a survey conducted after screening of this film, 93 percent of viewers said they were more aware about the dangers of skin cancer, and 82 percent said they would have their skin checked by a medical professional.
In addition to its commitment to diversity initiatives, Johnson and Johnson Consumer Health is also looking at ways to improve skin health for cancer patients. Results from a clinical study show that daily Avena Sativa (Oat) skincare regimen provides a significant improvement in xerosis and pruritus for adult patients undergoing systemic oncology treatments. Cancer treatment with kinase inhibitors often results in skin-related side effects, also known as Cutaneous Adverse Drug Reactions (CADRs), which can significantly impact patients’ quality of life.
About 90 percent of cancer patients have skin reactions, and if these adverse events are bad enough, it can have a significant impact on quality of life, Kizoulis explains, emphasizing the importance of finding ways to help these patients.
“The skin barrier is greatly impacted by certain cancer treatments. As the skin cells normal programming is altered, the skin barrier is damaged, allowing water to escape more easily and irritants to more easily penetrate the skin,” says Dr. Georgios Stamatas, Research Associate Director and Fellow, Johnson & Johnson Consumer Health. “Johnson & Johnson Consumer Health and Janssen Pharmaceutical Companies of Johnson & Johnson have shown with this latest research that we can significantly improve mild-to-moderate skin-related side effects with topical solutions, helping patients focus on their cancer treatment.”
Patients enrolled in the study reported common side effects of mild-to-moderate xerosis (µ=1.5) and pruritus (µ=0.9) related to their cancer treatment. Patients used an Avena Sativa Skincare Regimen for five weeks, which included:
- Aveeno’s Restorative Skin Therapy Sulfate-Free Body Wash used once-a-day for all showering. The body wash contains antioxidant oat, aloe, and pro-vitamin B5.
- Aveeno’s Restorative Skin Therapy Oat Repairing Cream applied twice daily. The body cream contains prebiotic oat concentrate, aloe, and pro-vitamin B5. It is formulated without parabens, fragrance, phthalates, and steroids.
- Aveeno’s Restorative Skin Therapy Itch Relief Balm applied as needed. The anti-itch balm is formulated with prebiotic oat concentrate, aloe, pro-vitamin B5, and pramoxine HCl. The targeted treatment is formulated without parabens, fragrance, phthalates, and steroids.
After five weeks, significant improvements (p<0.05) in xerosis (µ=0.5) and pruritus (µ=0.4) were observed according to dermatologist reported Common Terminology Criteria Adverse Events (CTCAE) scoring and Patient Reported Outcomes Common Terminology Criteria Adverse Events (PROCTCAE) scoring. The regimen was well tolerated and no serious product-related adverse events were observed.
Patients also reported significant improvements (p<0.05) in skin moisture with the regimen. They perceived significant improvements in their quality of life as measured by Skindex-16 scores including their symptoms, emotional, and functional impact.
ASLMS Announces New Officers
Henry H.L. Chan, MD, PhD, FRCP, is the new President of the American Society for Laser Medicine and Surgery (ASLMS).
Dr. Chan is currently the Honorary Clinical Professor and Honorary Consultant Dermatologist of the Division of Dermatology, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong, and Visiting Scientist (research staff) of the Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, Massachusetts, USA. Since 2005, he has served as an Associate Editor of Lasers in Surgery and Medicine. He is the recipient of numerous ASLMS awards and citations, including the Ellet H. Drake Memorial Award for his contributions to laser surgery.
“It is my greatest honor to serve as the 41st president of the American Society for Laser Medicine and Surgery,” Dr. Chan says. “I joined ASLMS as an international member over 20 years ago, and since then, I have attended every single Annual Conference. I have wonderful memories, not only learning new technology from key opinion leaders but more importantly, intellectual exchange and professional networking with colleagues from all parts of the world.“
Dr. Chan succeeds Thomas Rohrer, MD, who now serves as the Society’s Past President.
The 2021-22 ASLMS Executive Committee also includes:
- President-Elect Paul M. Friedman, MD
- Vice President Murad Alam, MD, MSCI, MBA
- Secretary Kristen M. Kelly, MD
- Treasurer E. Victor Ross, MD,
- Treasurer-Elect Omar A. Ibrahimi, MD, PhD
- Historian J. Stuart Nelson, MD, PhD
Other new members of the Board include:
- Representatives for Laser Surgery: Molly Wanner, MD, MBA and Macrene Alexiades, MD, PhD
- Early Career Scientist: Student Bonnie Carney, PhD
- Resident/Fellow Student: Ashaki Patel, MD.
CLOSE UP with Jonathan Silverberg, MD, PHD, MPH

Baricitinib, an oral selective Janus kinase inhibitor, improved the clinical signs and symptoms of moderate to severe atopic dermatitis (AD) in the 16-week, Phase 3 monotherapy studies, BREEZE-AD1 and BREEZE-AD2, but many AD patients require longer term use of systemic therapy. To see how well baricitinib holds up in the longer run, Jonathan Silverberg, MD, PHD, MPH, an associate professor of dermatology and the director of clinical research and patch testing and George Washington University School of Medicine and Health Sciences in Washington, DC, and colleagues, conducted an extension study of these two randomized clinical trials. Their findings appeared online in JAMA Dermatology. Here, Dr Silverberg explains what they found and why it matters for your patients.
Why is this topic important to study?
Jonathan Silverberg, MD, PHD, MPH: Atopic dermatitis is a chronic disease often requiring long-term use of systemic therapy. Previous studies showed good efficacy for baricitinib by 16 weeks in the BREEZE-AD1, BREEZE-AD2 and BREEZE-AD7 studies. However, some patients who achieve a good clinical response with baricitinib may need to stay on therapy for an extended period. In addition, some patients with more severe disease or chronic lesions may take longer to achieve clinical response. The BREEZE-AD3 study examined clinical efficacy of baricitinib treatment for 52 weeks beyond the initial 16 weeks of treatment in BREEZE-AD1 and BREEZE-AD2.
Describe the research and your findings.
Dr. Silverberg: Patients who were responders to baricitinib at week 16, i.e. achieved clear, almost clear or mild Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores in BREEZE-AD1, BREEZE-AD2 and BREEZE-AD7 were rerandomized to receive either baricitinib 2mg or 4mg over a 68 week period. Similarly, non-responders to baricitinib at week 16, i.e. moderate or severe vIGA-AD scores, were rerandomized to 2mg or 4mg baricitinib over a 68 week period. The study found that baricitinib 4mg and 2mg demonstrated good long-term efficacy on AD signs and symptoms for up to 68 weeks in adults.
What is the next step?
Dr. Silverberg: The results presented in this manuscript are from the first treatment period of BREEZE-AD3. There is also a second treatment period examining randomized withdrawal and down titration of baricitinib. The results of this sub-study are pending. In addition, baricitinib is currently approved in Europe to treat moderate-severe AD patients requiring systemic therapy. We await approval of baricitinib by the United States Food and Drug Administration in the next few months.
JAMA Dermatology study (Long-term Efficacy of Baricitinib in Adults With Moderate to Severe Atopic Dermatitis Who Were Treatment Responders or Partial Responders: An Extension Study of 2 Randomized Clinical Trials)
Abbvie’s JAK1 Inhibitor Measures Up in Two Phase 3 Studies of AD
Upadacitinib yields rapid and significant improvements in patients with moderate to severe atopic dermatitis, according to results from two Phase 3 clinical trials published online in The Lancet.
Patients who received upadacitinib showed reductions in all clinical disease measures, as well as in all itch-related outcomes and there were no unexpected safety signals.
“The results of these trials (Measure Up 1 and Measure Up 2) were so incredible that by week 16, most patients with moderate to severe atopic dermatitis either had a 90 percent disease clearance, or even 100 percent disease clearance,” says Emma Guttman-Yassky, MD, PhD, Waldman Professor and Chair of the Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, and first author of the paper.
“We achieved extremely high clearance rates that are bringing us closer to the amazing clearance rates that we see in psoriasis,” says Dr. Guttman-Yassky. “Also, the itch improvements already started to be significant within days from the beginning of the trials, and the maximum clinical efficacy was obtained early, at week 4, and maintained to week 16.”
If you look at what each of the JAK inhibitors are targeting, JAK1 tackles the important cytokines in atopic dermatitis—IL-13, IL-4, IL-31, and IL-22—and that’s why we’ve seen amazing results in atopic dermatitis,” she says.
She also notes that upadacitinib was well tolerated by patients in the two highest doses of the drug—15 mg and 30mg—and no important safety risks were observed.
“The results achieved with this study were very impressive and provided robust efficacy,” shares Dr. Guttman-Yassky. “Patients were able to start and restart the oral medication at any time, allowing for flexibility, which cannot be achieved with biologics.”
While biologics have been very successful for treating patients with atopic dermatitis for whom topical treatments have not worked or are not advised, Dr. Guttman-Yassky explains that they cannot be stopped and restarted at will because of a potential to create anti-drug antibodies, which will shorten the half-life of the drug.
Dr. Guttman-Yassky says that the study also provides the opportunity for adolescents and adult patients who fear injections the option of taking an oral systemic medication for their disease and is an alternative for those suffering from moderate to severe atopic dermatitis. “Right now, we have dupilumab, which is an amazing drug that has utterly changed the landscape of the disease. However not everybody responds to it, and because it’s an injectable medication, it’s not suitable for all patients,” she says.
The Measure Up 1 study took place between August 13, 2018 and December 23, 2019, and 847 patients were enrolled. The Measure Up 2 study took place between July 27, 2018 and January 17, 2020, and 836 patients were enrolled. The study was funded by AbbVie, Inc.
Inspire Patient Experience with Practical Dermatology®
Your Patients Are Talking About…Alcohol and Psoriasis

To help readers better understand the issues that patients with skin diseases face, Practical Dermatology® magazine partnered with Inspire to provide Inspire Insights. With more than 100 national patient organization partnerships and over two million members, Inspire is a vital social network that enables safe sharing of health experiences and social support.
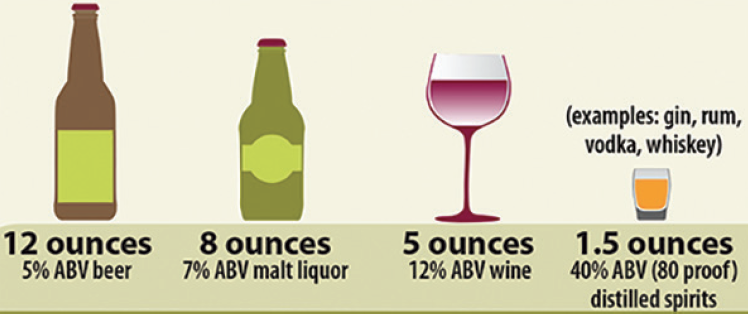
Photo credit: CDC
“I tried just wine and then just beer and then just liquor but the only thing that worked for me was no alcohol at all. I went from 70 percent covered to less than five percent covered,” says one Inspire member.
Another Inspire member shares, “Pure vodka from potato okay for me with tonic and drinking in moderation.”
About four percent of Inspire psoriasis members search for how alcohol affects their psoriasis flare-ups or psoriasis treatments.
“Alcohol is a well-known trigger of psoriasis because it can cause further production of proinflammatory cytokines,” explains Practical Dermatology® magazine’s Clinical Focus-Psoriasis editor Jennifer Soung, MD, a dermatologist at Southern California Dermatology in Santa Ana, CA. She is also the director of clinical research at Southern California Dermatology in Santa Ana and a clinical professor at Harbor–UCLA.
Her advice? “Drink in moderation. For women, this means one glass of wine per day, and for men, it’s two glasses of wine. “
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!



