Nitric Oxide-Releasing Cleanser for Plantar Warts
Nitric oxide (NO) is a ubiquitous endogenously produced gas that affects numerous physiological and cellular pathways including inflammation, immunomodulation, and vasodilation.1 In the skin, NO serves essential roles in wound healing and epidermal barrier function, and it defends against bacteria, viruses, and fungi.2-5 This antimicrobial effect is well known and concentration dependent; at low concentrations, NO is an immunomodulator, signaling immune cell growth, activation, and regulation via NF-kappaB regulation, cytokine, and apoptotic pathways.6 At higher concentrations, NO exerts direct broad spectrum antimicrobial static and cidal activity by covalently binding to pathogenic DNA, proteins and/ or lipids.6 Exogenously, its ability to target pathogenic bacteria, viruses, and fungi through multiple mechanisms without risk of antimicrobial resistance make it an attractive therapeutic candidate.7
Indeed, transdermal, sublingual, oral, intranasal, and intravenous NO have met with therapeutic success.4,5-10 NO has shown anti-viral activity against a broad range of dermal viral infections including molluscum contagiosum and human papillomavirus (HPV).10-13 Direct application of NO inactivates viruses by interacting with viral proteins, thereby rendering them inactive.4,13 NO is a small molecule that easily permeates dermal tissues, and efforts to harness topical forms of NO for the treatment of dermatological viral conditions have gained momentum.10-17 The recent FDA approval of berdazimer topical gel lends credence to the antimicrobial activity of topically delivered NO for the management of molluscum contagiosum virus.18 Previous studies have addressed NO’s effectiveness against HPV.12,13,19 Other forms of NO have been investigated for dermatological infections including acidified nitrites.19-25 Plantar warts (Verruca plantaris), caused by HPV, are common and appear on the plantar region of the foot.26,27 Although the condition is benign and self-limiting, there is risk of autoinoculation and spread to others through skinto- skin contact or contamination of surfaces while lesions are present.26,27 Often painful because of constant pressure and friction, plantar warts may impair gait, ambulation, participation in sports, and wearing of shoes.27 Bleeding, secondary infection, and stigmatization are also common. Despite a number of plantar wart treatments, none has been shown to be highly effective.27,28 Cryotherapy and high concentration salicylic acid, both first-line treatments, are painful, have lowcure rates, and/or must be administered in-office.29 Topical NO-releasing formulations are being explored as a viable therapeutic option to help relieve plantar warts.
CASE VIGNETTE
A 12-year-old girl developed a few small warts on the plantar surface of both feet, and over a 6-month period, they expanded in both number and size, spreading over the soles and great toes. The condition caused extreme pain Nitric Oxide-Releasing Cleanser for Plantar Warts Cryotherapy and high concentration salicylic acid, both first-line treatments, are painful, have low-cure rates, and/or must be administered in-office. Topical NO-releasing formulations are being explored as a viable therapeutic option to help relieve plantar warts and adversely affected gait; she had not responded to three courses of topical liquid nitrogen nor to salicylic acid treatments. (Figure 1, Baseline Day 1). The patient was instructed (with parental oversight) to self-treat the lesions at home with a 5-liter, warm foot bath containing a non-prescription NO-releasing solution (NORS) for 30 minutes, three times per week, with 1 to 2 days between treatments for 2 weeks. A total of six treatments were targeted over the 2-week period. Salicylic acid was used during some treatments. Improvement was seen by Day 7 with painless ambulation after 48 hours and complete resolution at Day 28 (Figure 1). The foot bath was well tolerated and effective for this patient; she found the foot bath was easy to use, soothing, and without an unpleasant odor. There was no recurrence of plantar warts.
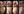
FIGURE 1: improvement of the appearance of plantar warts with use of a NORS foot bath cleanser.
CLINICAL IMPLICATIONS
A 12-year-old girl developed a few small warts on the plantar surface of both feet, and over a 6-month period, they expanded in both number and size, spreading over the soles and great toes. The condition caused extreme pain and adversely affected gait; she had not responded to three courses of topical liquid nitrogen nor to salicylic acid treatments. (Figure 1, Baseline Day 1). The patient was instructed (with parental oversight) to self-treat the lesions at home with a 5-liter, warm foot bath containing a non-prescription NO-releasing solution (NORS) for 30 minutes, three times per week, with 1 to 2 days between treatments for 2 weeks. A total of six treatments were targeted over the 2-week period. Salicylic acid was used during some treatments. Improvement was seen by Day 7 with painless ambulation after 48 hours and complete resolution at Day 28 (Figure 1). The foot bath was well tolerated and effective for this patient; she found the foot bath was easy to use, soothing, and without an unpleasant odor. There was no recurrence of plantar warts.
LOOKING AHEAD
Topical NO is a potent, multi-mechanistic antimicrobial molecule with potential utility against several dermatological infections without the risk of antimicrobial resistance. NO’s ability to diffuse across most physical barriers due to its lipophilic and hydrophilic properties overcomes challenges to targeted cutaneous delivery in dermatological infections.3 The availability of at-home NO-releasing products lends to patient convenience. Exploratory observations using other formulations of NO-releasing products are underway for onychomycosis, tinea pedis, and other skin infections.
Gilly Regev, PhD
- Entrepreneur, Founder, CEO, SaNOtize Research and Development Corporation; Vancouver, BC
Christopher Miller, PhD
- Co-Founder, Chief Scientific Officer, SaNOtize Research and Development Corporation; Vancouver, BC
Martina Cartwright, PhD
- Senior Executive, Medical Affairs, Novan, Inc.; Durham, N.C.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!
Recommended
- Clinical Case Reports
Upadacitinib Therapy in a Pediatric Patient with Infliximab-Induced Skin Eruption
- Clinical Case Reports
Orchiectomy-Induced Melasma: Considering Melasma in Male Patients Post-Orchiectomy










