Atopic dermatitis (AD) is a chronic pruritic inflammatory skin disease that affects patients of all ages, particularly children. The impact on the quality of life (QoL) of those with AD is enormous, with unrelenting flares causing anxiety, depression, sleep disturbances, attention difficulties, and social withdrawal, to name a few.1 The pathogenesis of AD is not fully elucidated, but it is clearly a multifactorial disease involving skin barrier dysfunction, immune dysregulation, microbiome imbalance, as well as a neuro-behavioral component.2 While there are numerous conventional therapies, both topical and systemic, such multitudes conjure the insightful axiom: “In general, successful treatment of any disease is probably inversely related to the number of recommended regimens.”3 Conventional approaches include topical corticosteroids, calcineurin inhibitors, antibiotics, immunosuppressants, and most recently a biologic agent, with many more in development. While generally effective, these treatments are not without side effects, and increasing numbers of patients are seeking alternative and complementary therapies perhaps due to both real and perceived risks.

This “toolbox” for AD treatments outlines an approach for practitioners to consider a combination of therapies from different traditions from botanicals, to acupuncture, to phototherapy. Furthermore, it hopes to encourage health care providers from all traditions to work together to support different aspects of AD treatment. This toolbox is organized into five “pillars” of AD: skin barrier, psyche, inflammation, microbiome, and itch. The treatments discussed are practical (attainable, simple, not requiring prescriptions), safe (reasonable safety evidence or long experience when such information is lacking), and effective (evidence-based, though almost never to the standards of conventional medications, otherwise they would arguably be part of the mainstream approach already). While not exhaustive, the hope is to stimulate discussion and inspire further explorations into an integrative approach for treating AD.
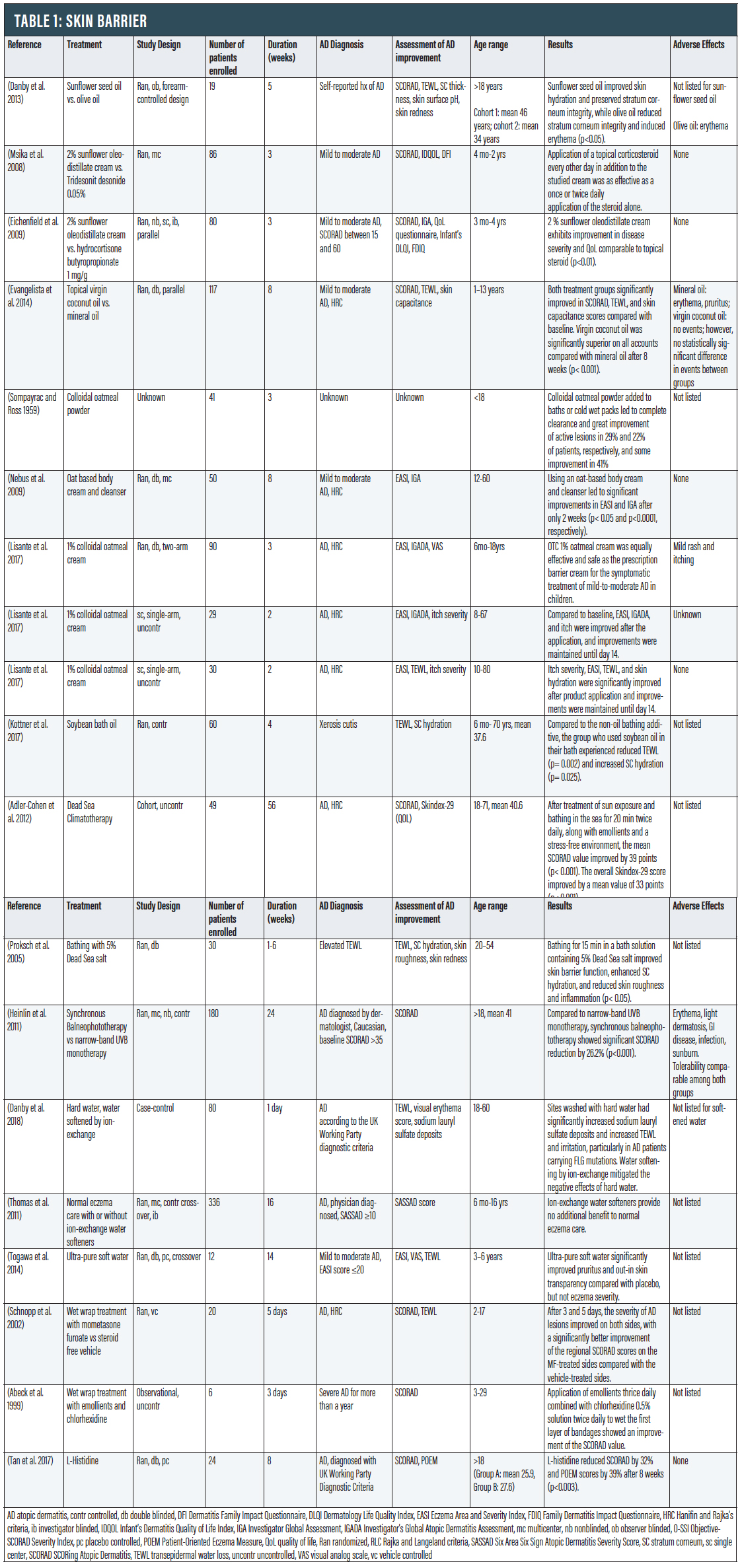
Skin Barrier
Dry skin and impaired skin barrier are defining characteristics of AD. The most superficial layer of the skin consists of keratinocytes, structural proteins such as filaggrin, and lipids.4 Filaggrin proteolysis releases L-histidine and other amino acids that maintain skin hydration by acting as natural moisturizing factors to attract moisture. Lower levels of natural moisturizing factors are associated with transepidermal water loss (TEWL) and have been implicated in AD pathogenesis. This defective skin barrier increases immunogen and pathogen entry, resulting in increased inflammation and exacerbation of disease. It is thus very important to maintain an intact skin barrier to control AD. Moisturizer is a mainstay of treatment and adding plant oils on top may have additional therapeutic benefits.
Sunflower Oil. Natural oils have been used in skincare for centuries. A number of natural oils with beneficial fatty acids can help repair the natural skin barrier, but not all oils are good for the skin; some can be irritating and even worsen AD. The plant source, composition, and oil extraction process are important factors in determining their benefit. Cold pressed oils (also known as virgin oils) are made by extracting oil from seeds or nuts without adding chemicals or heat and tend to be favored for skin use. Sunflower oil is cold pressed from the seeds of the sunflower and has long been used as an emollient in cosmetic formulations.
Sunflower oil can be used topically to increase synthesis of ceramides and has direct emollient and barrier repair properties. It contains a high level of linoleic acid, a long chain fatty acid and a major constituent of the barrier lipids that can improve the skin barrier and yield anti-inflammatory effects.5 Stratum corneum (SC) is the outermost layer of the epidermis and has shown to be impaired in AD skin.6 When compared with topically applied olive oil, sunflower seed oil was found to improve skin hydration and preserve stratum corneum integrity, while olive oil significantly reduced stratum corneum integrity and induced erythema (p<0.05).7
Sunflower Oleodistillate (SOD) cream has also been studied for its potential steroid-sparing effects. This active substance is extracted from an edible cooking oil by molecular distillation, a physical process free of solvents, that yields an extract whose content is enriched 10-fold in its unsaponifiable fraction (phytosterols and vitamins) compared to the edible oil. SOD restores skin barrier functions, specifically replenishing essential key lipids.8 Eighty‐six children with moderate AD were randomized to receive daily topical corticosteroids or every other day corticosteroids alternating with emollient containing 2% SOD. After three weeks, all groups had similar significant reductions in the Scoring Atopic Dermatitis scale (SCORAD), and the group treated with SOD alternating with corticosteroid had statistically significantly greater improvement in lichenification and excoriation than the corticosteroid alone group (p<0.1).8 Another randomized trial of 80 children with mild to moderate AD found that a 2% SOD cream exhibited significant improvement in disease severity and QoL comparable to a corticosteroid at days seven and 21 of treatment (p<0.01).9
Coconut Oil. Coconut oil is derived from the white lining within the shell of mature coconuts. Virgin coconut oil (same as extra virgin in this context) has demonstrated both emollient and antibacterial properties, making it an appealing therapy for AD. Virgin coconut oil is obtained by a wet‐milled, cold‐press process that does not involve the addition of chemicals or heat.10 This absence of refining, heat, and solvent extraction preserves the active components of coconut oil (antioxidants and fatty acids).11 In a study including 117 patients, virgin coconut oil was significantly superior to mineral oil in SCORAD and TEWL assessments (p< 0.001), though both groups found significant improvement compared with baseline.12
Bathing additives. Bathing additives are often used for a range of inflammatory dermatoses and can be useful adjuvant treatments for eczema. The benefits of bathing additives are multifactorial, including anti-inflammatory, antimicrobial, antioxidative, and skin repair.
Oatmeal. Colloidal oatmeal compounds became commercially available for the treatment of inflammatory skin conditions in 1945.13 In 2003, the FDA approved colloidal oatmeal as a “skin protectant,” meaning the agency consider it effective for alleviating dryness, itching, and discomfort caused by certain skin conditions, including AD.14 Colloidal oatmeal is uncooked oatmeal that has been ground into a fine, consistent powder using a blender or food processor and can be added to water to form a smooth, milky liquid that can be used for baths or wet packs to relieve dry, itchy, irritated skin caused by AD. Oats contain hydrophilic carbohydrates that enhance moisture uptake and retention, as well as unsaturated triglycerides, flavonoids, tocols, alkaloids, and sterols that further exert barrier repair and anti-inflammatory effects.13 Furthermore, oats contain vitamin E and ferulic acid that have antioxidant and anti-inflammatory properties.15
In a Sompayrac and Ross study involving 41 pediatric patients with mild to severe AD, colloidal oatmeal powder (46 percent oat starch, 24 percent oat protein, nine percent oat oil, 0.03 percent crude fiber, eight percent moisture) added to baths or cold wet packs led to complete clearance and great improvement of active lesions in 29 and 22 percent of patients, respectively, and some improvement in 41 percent after one to three weeks of treatment.16 These findings show promise in the use of oatmeal-based bathing additives in AD; however, the study was limited by the concurrent use of other unspecified topical treatment modalities. Colloidal oatmeal can be found in creams.
In a study involving 50 pediatric and adult patients with AD, using an oat-based body cream and cleanser led to significant improvements in Eczema Area Severity Index (EASI) (p< 0.05) and Investigator’s Global Assessment (IGA) after only two weeks (p<0.0001).17 Over-the-counter 1% colloidal oatmeal cream was found to be equally effective and safe as a prescription barrier cream in 90 children with mild to moderate AD.18 Another two studies with both pediatric and adult AD populations (n=29 and n=30, respectively), showed improvements in itch intensity, TEWL, and skin hydration after application of 1% colloidal oatmeal cream that were maintained for two weeks.19
Bathing oils. Adding topical oils to a moisturizing routine has potential benefits to improve the skin barrier and reduce water loss; utilizing oils as a bathing additive can provide additional benefit. Both plant- and mineral-derived oils function as emollients to protect and hydrate the skin.20 In a 28-day RCT, 60 healthy children and adults with xerosis cutis either bathed with Balneum Hermal (85 percent refined soybean oil) for 20 minutes every other day or continued their regular skin cleansing practice with a non–oil-containing cleanser or bath additive (control group). Participants in the oil bath group had significantly reduced TEWL (p= 0.002) and significantly greater SC hydration (p= 0.025), compared with controls. While some guidelines indicate evidence is insufficient for their recommendation, there is likewise insufficient evidence definitively showing that they do not help.21 One notable adverse event related to bath oils is the risk of slipping in baths or showers.
Bath Therapy (Balneotherapy). The ancient practice of balneotherapy re-emerged as a treatment modality in the 1800s in Europe.22 Immersion in mineral water baths or pools is a potential remedy to increased TEWL.23 For centuries, the healing powers of the Dead Sea have attracted people with a wide variety of ailments. Dead Sea salt solution has high magnesium content, which is known to bind water, influence epidermal proliferation and differentiation, and enhance permeability barrier repair.23 A clinical trial of 49 AD patients treated at the Dead Sea (gradually increasing sun exposure, bathing in the sea for 20 minutes twice daily, plus emollients and a stress-free environment) found a significant improvement in the SCORAD index (p< 0.001) and QoL. Though a vacation at the Dead Sea isn’t possible for most patients, several attempts have been made to recreate those conditions.24 In a study of patients with elevated TEWL, bathing for 15 minutes in a solution containing 5% Dead Sea salt was well tolerated, improved skin barrier function, enhanced stratum corneum hydration, and reduced skin roughness and inflammation (p<0.05).25 Furthermore, in a RCT of 180 AD patients, synchronous balneophototherapy—a simulation of Dead Sea treatment conditions via mineral bath content and ultraviolet light exposure—significantly reduced AD severity when compared with narrow-band UVB monotherapy (NB-UVB) (p< 0.001). This suggests that the addition of hypertonic, magnesium rich baths to phototherapy confers additional benefit.
Wet Wraps.Wet wraps are a common short-term treatment for AD that can improve the epidermal barrier, increase water content in the skin, and act as a physical barrier from scratching.26 The concept of wet-wraps includes applying topical medication and/or moisturizer to damp skin, then applying a damp layer of clothing or gauze, followed by a dry layer of clothing or gauze and leaving in place for at least several hours. For people who do not want as many layers, “modified wet wraps” omit the final dry layer of clothing/gauze.27
A 2006 review of the literature concluded that wet wraps are an effective short-term treatment in children with severe or refractory AD, and pairing the wraps with a topical corticosteroid is more effective than using emollients alone.28 One randomized vehicle-control trial included a wet-wrap treatment on both arms of 20 patients, using mometasone furoate (MF) ointment on one side and its steroid-free vehicle on the other side.29 After three and five days, the severity of AD lesions improved on both sides, with a significantly better improvement of the regional SCORAD index on the MF-treated sides compared with vehicle-treated sides. Another clinical study detailing wet-wrap therapy with application of emollients thrice daily combined with chlorhexidine 0.5% solution twice daily to wet the first layer of bandages showed an improvement of SCORAD.30 Adverse events resulting from wet wraps are not common and usually mild.28 The most common adverse effects were discomfort (chills) and folliculitis.
L-Histidine Supplementation. Filaggrin is a key structural protein in the epidermis and critical for maintenance of the epidermal barrier.31 Mutations in the filaggrin gene are strongly associated with AD. Filaggrin also plays an important role in maintaining skin hydration. In mammalian skin, L-histidine is rapidly incorporated into filaggrin.32 Subsequent filaggrin proteolysis releases L-histidine as an important natural moisturizing factor. Studies have evaluated L-histidine supplementation as a way to increase filaggrin synthesis. In vitro studies using human keratinocytes demonstrated L-histidine significantly increased both filaggrin formation and skin barrier function. Furthermore, a clinical trial showed that L-histidine supplementation (4g/d) in adults with AD significantly reduced AD severity by 34 percent (SCORAD) with a similar effect to a mid-potency topical steroid (p<0.003) with no adverse effects (p<0.003).32
Gut Barrier Repair. Impairment of the skin barrier in AD patients is well known, and it has been postulated that the intestinal barrier is also compromised.33 Alteration of the equilibrium in the gut barrier leads to the passage of the luminal contents to the underlying tissues and the bloodstream, resulting in activation of the immune response and the induction of gut inflammation.34 Implication of the gut in AD has been persistent, with many insisting diet is the “root cause.”35 A study from the United Kingdom identified significantly increased intestinal permeability (“leaky gut”) in children with AD when compared to children without AD, while another found a relationship between the extent of gut leakiness and AD severity.36 Thus, interventions to improve the gut barrier may treat the skin as well.
Gelatin tannate is a complex of tannic acid that possesses astringent, antibacterial, and anti-inflammatory properties. It can function as a demulcent, forming a protective layer with mucin in the mucus layers of the gut to improve the gut barrier.34 Additionally, it appears to act as an astringent and helps to eliminate inflammatory proteins.37 Such improvements to the “internal” epithelium may lead to improvements to the external epithelium by modulating the immune system and reducing inflammation.34 This may prove to be a viable option for improving gut barrier function, perhaps akin to a topical moisturizer for the skin, but more studies are needed. (Download this article from PracticalDermatology.com to access summary tables for each section.)
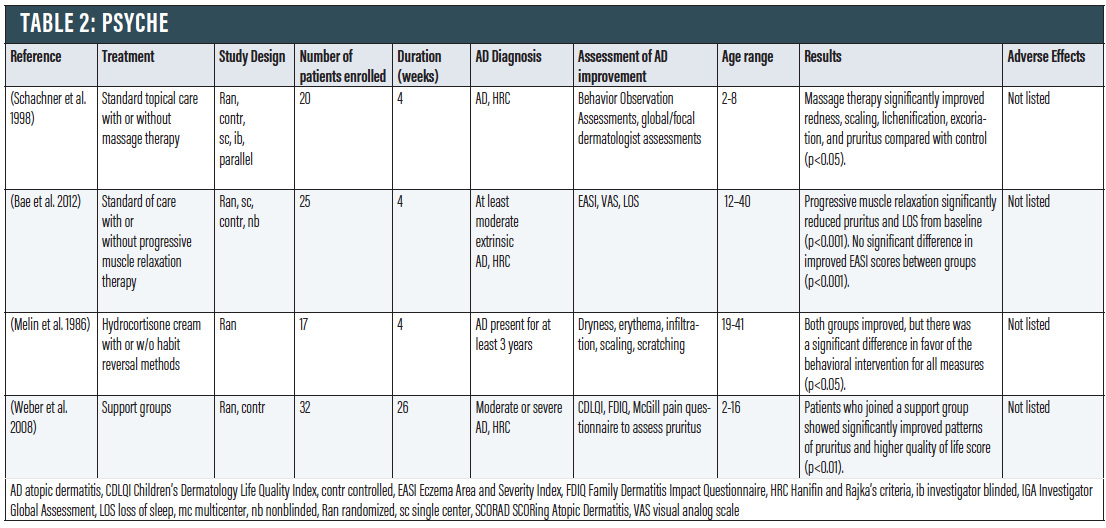
PSYche
Stress and anxiety appear to play an important role in worsening AD symptoms.38 Vicious cycles can form where stress and anxiety from AD can lead to further scratching and itching, which can lead to more stress and anxiety. Thus, it is advantageous to adapt stress management techniques to address the importance of psyche in AD.
Stress Management. When compared to standard topical treatment, massage therapy significantly improved redness, scaling, lichenification, excoriation, and pruritus in 20 children ages two to eight (p<0.05).39 Massage therapy also appears to increase peripheral blood flow and relieve anxiety associated with AD. In a trial of 25 patients that examined standard of care with or without progressive muscle relaxation (PMR) therapy, the degree of pruritus and loss of sleep was significantly decreased in the PMR group (p < 0.001), but not among controls.40 Another RCT applying a behavioral method of habit reversal to scratching in AD patients in combination with hydrocortisone cream compared to hydrocortisone cream alone showed a significant difference in favor of the behavior intervention (p<0.05).41 Finally, patients who joined a support group to cope with stress showed significantly improved patterns of pruritus and higher QoL score (p<0.01).42
Though evidence is limited, it appears that stress-relieving techniques can at least play an adjunctive role in managing AD. Furthermore, sleep disruption continues to be a major problem for many patients, and the notion that something safe and inexpensive like progressive muscle relaxation could be of value is compelling and worthy of more research.
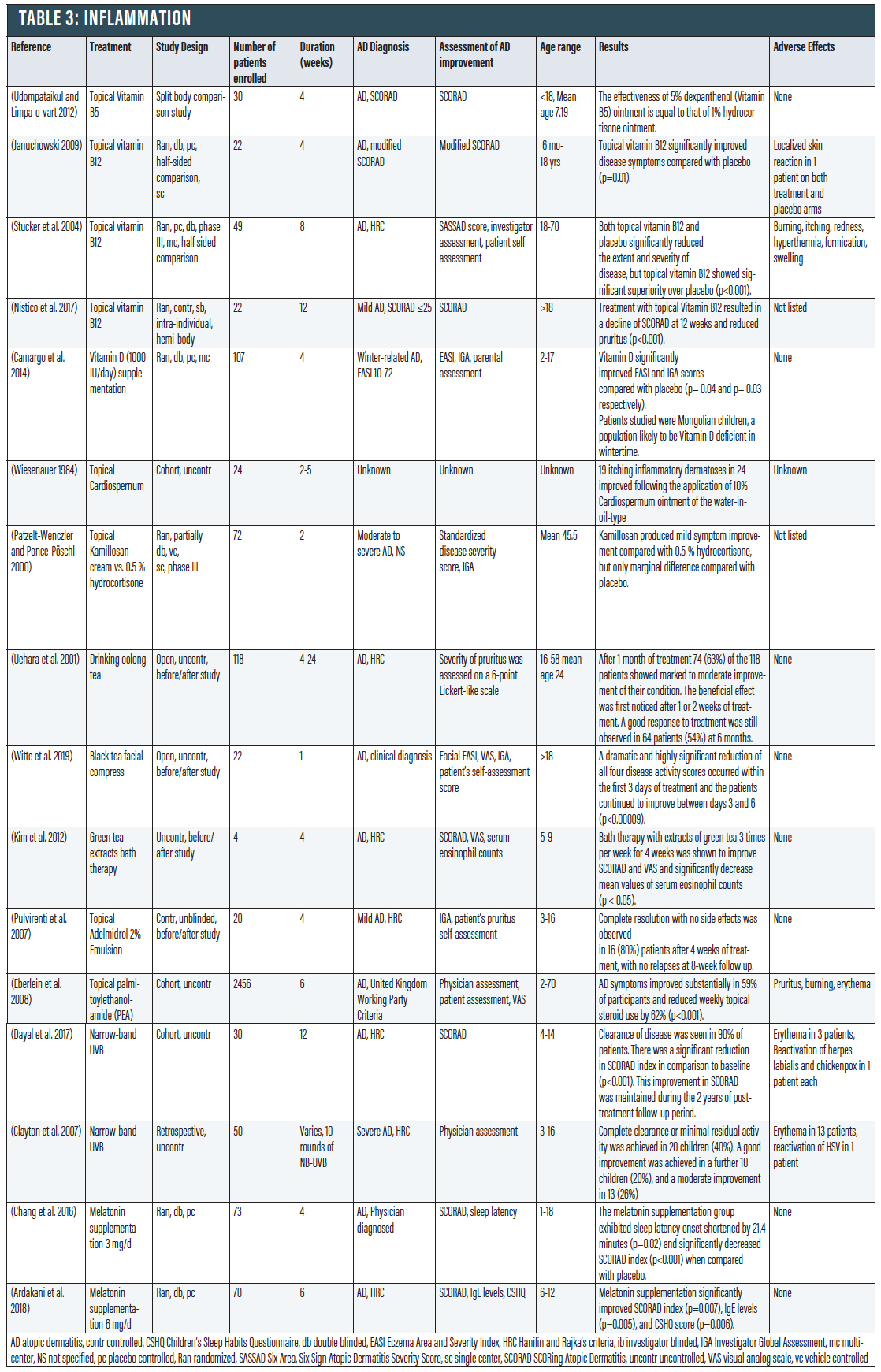
Inflammation
While topical corticosteroids, currently the mainstays of treatment for AD, are effective, the risks of inappropriate topical corticosteroids (including atrophy, striae, dyspigmentation, and hypertrichosis) must be weighed against their benefits. These risks give further motivation to find non-steroidal treatments for AD. Several topical vitamins hold promise in alleviating AD symptoms.
Vitamin B5. Vitamin B5 encourages wound healing by inducing keratinocyte proliferation and increasing glutathione antioxidant in the skin.43 A study concluded that dexpanthenol 5% (Vitamin B5) ointment might be equally efficacious as hydrocortisone 1%, and may be used as an alternative therapy in children with mild to moderate AD.44
Vitamin B12. Topical Vitamin B12 (0.07%) can help prevent AD flares.45 It has been thought to work via inhibiting nitric oxide synthase and is thought to attenuate eczema-related symptoms by reducing nitric oxide levels.45 In a study with 22 pediatric patients, topical Vitamin B12 was shown to significantly reduce AD symptoms compared to placebo (p=0.01).46 Furthermore, topical Vitamin B12 was also shown to be effective in reducing AD symptoms in a study with 49 adults (p<0.001). In another intra-individual split body RCT involving 22 mild AD patients, the side of the body treated with MB12 (Vitamin B12-barrier cream) two to three times per day for 12 weeks had improvement in SCORAD and decrease in pruritus from baseline compared to the side treated with the control glycerol–petrolatum-based emollient cream (p < .001).47
Vitamin C. Vitamin C has antioxidant benefits, and topical Vitamin C has been used to fight free radical damage, increasing collagen synthesis, and decreasing hyperpigmentation.48 It was found that AD patients have a lower oxidative repair ability associated with significantly lower serum levels of vitamin C than those without AD.49 Another study using a mouse model of house mite-induced AD showed that daily application of vitamin C-zinc oxide complex cream for six days reduced mast cells and significantly decreased expression of pro-inflammatory IL-4 and chemokine ligand 4 (CCL4) compared with the control group.50
Oral Vitamin D. Though topical Vitamin D is not recommended, and studies have shown that it can actually exacerbate AD symptoms, oral Vitamin D is a different story.51 There is compelling evidence to suggest lower Vitamin D levels are correlated with more severe AD, and Vitamin D supplementation can provide benefits to AD patients. Studies in children have found that higher vitamin D levels are significantly associated with decreased AD severity and SCORAD.52 Its role in AD may lie in its widespread effects on immune reactivity and skin barrier integrity.53 A study looked at children with winter-related AD and postulated that worsening AD is due to decreased Vitamin D from less sunlight.54 These children showed significant improvement with vitamin D supplementation versus placebo (p<0.04).
Botanicals. Topical botanicals have been used for centuries for various skin ailments, and AD is a potential indication for topical herbal anti-inflammatory drugs. Among them is Cardiospermum halicacabum, a member of the Sapindaceae family. Comparative blind trials using Cardiospermum for AD do not exist; it may have anti-inflammatory activity. In an uncontrolled trial, 19 itching inflammatory dermatoses in 24 improved following the application of 10% Cardiospermum ointment of the water-in-oil-type.55 Reports of unwanted effects including contact allergy are virtually absent.
Chamomile contains lipophilic elements, such as essential oils and coumarins, as well as lipids including wax-like substances.56 Chamomile is also believed to have anti-inflammatory effects, as a study in 1992 showed that camomile extract in vitro inhibits the production of prostaglandins and leukotrienes. The Manzana type of chamomile is rich in active ingredients and does not exhibit chamomile-related allergenic potential. In a partially double-blind, randomized study carried out as a half-side comparison, a chamomile extract cream was found to be slightly more effective than 0.5% hydrocortisone cream.57
Tea. Drinking oolong tea has also been used to treat inflammation caused by AD due to the antiallergic properties of tea polyphenols.58 In a study, 63 percent of patients who drank oolong tea BID for a month showed improvement of their condition, with the beneficial effect first noticed after one or two weeks of treatment.
Wet dressings with black tea could offer an effective, well-tolerated and low-cost treatment for facial AD.59 Black tea is known to contain astringent substances and flavonoids that both possess anti-inflammatory properties, and a water-based dressing is known to reduce acute epidermal inflammation. A black tea compress is composed of a soft cloth soaked in weak black tea and applied to the affected area for 20 minutes. When this compress was done five times a day, patients in a study showed significant decrease in AD symptoms (p<0.00009).60
In addition to its antioxidant properties, green tea also has anti-inflammatory properties and has been thought to be a potential safe and effective AD treatment.61 In a pilot study, bath therapy with extracts of green tea three times per week for four weeks was shown to improve SCORAD and significantly decrease mean values of serum eosinophil counts (p<0.05).62
Cannabinoids. Cannabinoids without psychoactive effects have the potential to treat AD topically via anti-inflammatory, anti-itch, and pain-relieving properties.63 Human trials using cannabinoids have shown promising outcomes. In one pilot study, a topical cannabinoid emulsion (Adelmidrol 2%) resulted in complete clinical resolution and prevented relapse of mild atopic dermatitis in 80 percent of patients.64 Another uncontrolled trial utilized endocannabinoid palmitoylethanolamide (PEA), which has antipruritic properties when applied topically. After four to six weeks of using PEA, 59 percent of 2,456 people with AD (both adults and children) demonstrated reduced pruritus and improved dryness, excoriation, lichenification, scaling, and erythema (p<0.001); 62 percent of participants also noted decreased weekly topical steroid use.65
Phototherapy. Phototherapy is thought to be anti-inflammatory by a number of mechanisms, including immunomodulation via lymphocyte apoptosis, depletion of Langerhans cells in the skin, and suppression of proinflammatory cytokines.66 NB-UVB (311-313 nm,) is often considered the first-line phototherapy, owing to its efficacy, availability, ease of administration, and minimal side-effect profile.67 It is longer-lasting and less erythemogenic than Broadband UVB (290-320nm) due to the exclusion of short wavelength UVB irradiation.68 NB-UVB has also been shown to be effective in replenishing Vitamin D deficiency, which may also play a role in AD.69
In a study with 30 children with AD, NB-UVB administered twice weekly for 12 weeks led to a significant reduction in SCORAD (p<0.001); improvement remained for at least two years, suggesting there may be a remittive effect.70 Clearance of disease was seen in 27 patients. Another retrospective study with 50 children showed that NB-UVB was effective in improving or completely clearing AD in 86 percent of patients and that clearance was seen even in more severe AD.71
Chronotherapy/Circadian Rhythm. Finally, both what and when anti-inflammatory treatments are administered are important. Circadian rhythm has important implications in AD because it influences cutaneous blood flow, skin immune function, and properties of skin barrier function, such as TEWL and skin capacitance.72 Chronotherapy is the practice of delivering drugs in synchrony with the circadian rhythm cycles of a condition or symptoms to deliver drugs at optimal time points to maximize therapeutic benefits and minimize adverse effects.73 For example, because TEWL and skin blood flow are highest in the evening, moisturizers and topical anti-inflammatory drugs should be applied at that time for greater penetrance and to impart maximum therapeutic benefits.74 In addition, the administration of topical and systemic treatments that modulate the immune system may be given at the same time as the endogenous morning cortisol surge to allow for synergistic anti-inflammatory effects.75
Melatonin. AD patients often have poor sleep quality due to pruritus; sleep disturbances can perpetuate AD by shifting the immune milieu further toward a Th2 response.76 This creates a cycle of AD exacerbations and poor sleep. Therefore, addressing sleep disturbances should be an integral component of the AD treatment plan. Melatonin is a major neurohormone that regulates the circadian rhythm, increases sleepiness, lowers core body temperature, and decreases cutaneous inflammatory markers associated with AD, such as IL-4 and immunoglobulin E.77 AD patients have lower circulating melatonin levels during periods of exacerbation.78 Conversely, higher nocturnal melatonin is associated with less sleep disturbance and less severe AD symptoms in children.79 Melatonin may affect AD by decreasing wake time at night, reducing scratching opportunities, and modulating the immune system, and can be considered as an adjunct therapy for AD patients. In a RCT, AD children who received melatonin supplementation (3mg daily for four weeks) exhibited sleep latency onset shortened by 21.4 minutes and significantly decreased SCORAD when compared with placebo.80 Another RCT with 70 children showed that melatonin supplementation significantly improved SCORAD index, IgE levels, and sleep quality.81
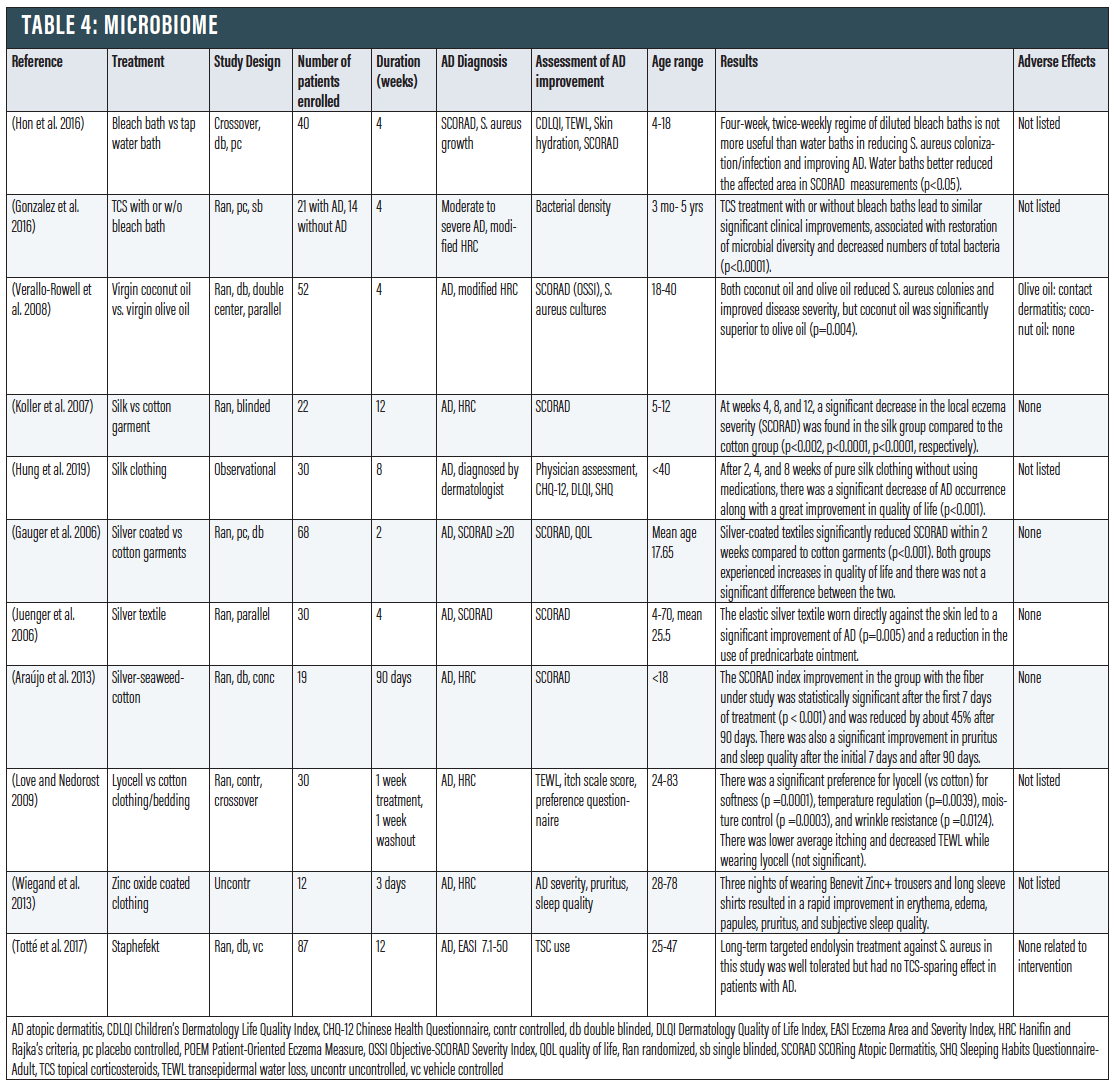
Microbiome
It has been convincingly demonstrated that the microbiome of patients with AD is not just a bystander, but can actually drive the disease. Defects in the skin barrier of those with AD allow potentially pathogenic bacteria to pass through more easily.82 People with AD have higher Staphylococcus aureus density on their skin, which can trigger inflammation and damage the skin barrier. Furthermore, those with AD do not produce sufficient antimicrobial peptides that normally contribute to keeping S. aureus levels at bay. This increases the risk for both S. aureus colonization and infection in those with AD.83
Bleach Baths. The use of dilute 0.005% bleach bath (0.5 cup of 6% common household bleach in a standard bathtub full of water) has been reviewed for the improvement of clinical symptoms of AD.84 Bleach has been postulated to improve AD through a combination of antimicrobial/antibiofilm,85 anti-inflammatory,86,87 and antipruritic87 effects. However, more recent studies have shown that the efficacy of bleach baths compared to a placebo may be less robust than previously thought. A cross-over trial with 40 pediatric patients concluded that a regime of diluted bleach baths is not more useful than water baths in reducing S. aureus colonization/infection and improving AD (p<0.05).88 Another RCT in 21 children with that showed that a topical corticosteroid with or without bleach baths led to similar significant clinical improvements that were associated with restoration of microbial diversity and decreased numbers of total bacteria, suggesting that the bleach baths did not add additional antimicrobial effect (p<0.0001).89
A small pilot study comparing dilute bleach soaks against apple cider vinegar soaks concluded that while bleach or apple cider vinegar may be effective treatments for AD, the mechanism of either solution is likely not due to antibacterial properties.90 Finally, a meta-analysis of five studies noted that although bleach baths are effective in decreasing AD severity, they do not appear to be more effective than water baths alone.91
While the jury is still out, a recent article summarizes the data to conclude: “These observations are not intended to directly support or refute the potential benefits of bleach bath therapy but only to dispel the false conclusion that it is a form of antimicrobial therapy. Indeed, because a recent meta-analysis concluded that water baths alone can significantly decrease the severity of atopic dermatitis, beneficial effects can be due to effects other than those acting directly on the skin microbiome.”92
Coconut Oil. The emollient properties of coconut oil have been shown to protect the skin barrier (see above). In addition, coconut oil potentially has antibacterial properties against S. aureus. A RCT with 52 patients showed that topically applied coconut oil decreased S. aureus colonization by 95 percent in patients with AD when applied twice daily for four weeks, vs. 50 percent decrease for olive oil control (p=0.004).93 It has been hypothesized that after lipase hydrolysis, virgin coconut oil produces medium chain fatty acids (mostly C-12) that are able to disrupt the bacterial cell membrane.94,95
Silk clothing. It is known that people with AD should avoid coarse wool clothing. Instead, cotton clothing is often recommended. There may be superior clothing alternatives. Silk clothing not only has a smooth surface to minimize friction against the skin, but also has been shown to have potential antimicrobial and anti-inflammatory properties. When 22 children with AD were given either a special silk garment or a cotton garment to wear over the cubital region,96 at weeks four, eight, and 12, a significant decrease in the local eczema severity (SCORAD) was found in the experimental group (p<0.002). Among 30 patients (both pediatric and adult), after two, four, and eight weeks of using pure silk clothing without using medications, there was a significant decrease of AD occurrence along with a great improvement in QoL (p<0.001).97
Silver Clothing. Silver-coated textiles have been shown to have an antibacterial effect on S. aureus.98 A study of 68 patients with AD compared silver-coated clothing to cotton garments and found that there was significant improvement in the SCORAD index in the silver-coated group within two weeks (p<0.001).99 Another study with 30 patients found that individuals using silver-coated clothing had significant reduction of eczema severity, as well as decrease in the use of a topical corticosteroid.100 Finally, a randomized double-blind controlled study of 19 children with AD compared placebo clothing to a new textile made of “silver-seaweed-cotton” fibers. By day 7 there was significant improvement in the SCORAD in the treatment group (p < 0.001), and that by day 90, there was 45 percent decrease in SCORAD as well as reduced pruritus and improvement in sleep quality.101
Endolysin. Endolysins are enzymes used by bacteriophages to degrade the peptidoglycan of the bacterial host from within, rupturing the cell.102 An OTC moisturizer, GladSkin that contains endolysin Staphefekt can target S. aureus and has been discussed for AD patients.103 One study with 87 adults using Staphefekt for 12 weeks showed that Staphefekt treatment was well tolerated but had no topical corticosteroid sparing effect in patients with AD. However, concurrent application of TCSs, emollients, or both may have masked a clinical benefit. Further studies are required to explore this potential option.
Itch
AD is sometimes referred to as “the itch that rashes.”104 The itch from AD can severely affect QoL, and it is of central importance to the pathogenesis of AD. Chronic itch in AD often precipitates sleep disturbance, attention difficulties, and social withdrawal, all contributing to a decreased QoL of affected individuals.105 Because pruritus is highly related to the other pillars of AD, other treatments have already been discussed that would help reduce pruritus. However, there are also several other alternative therapies that may benefit atopic patients in reducing itch.
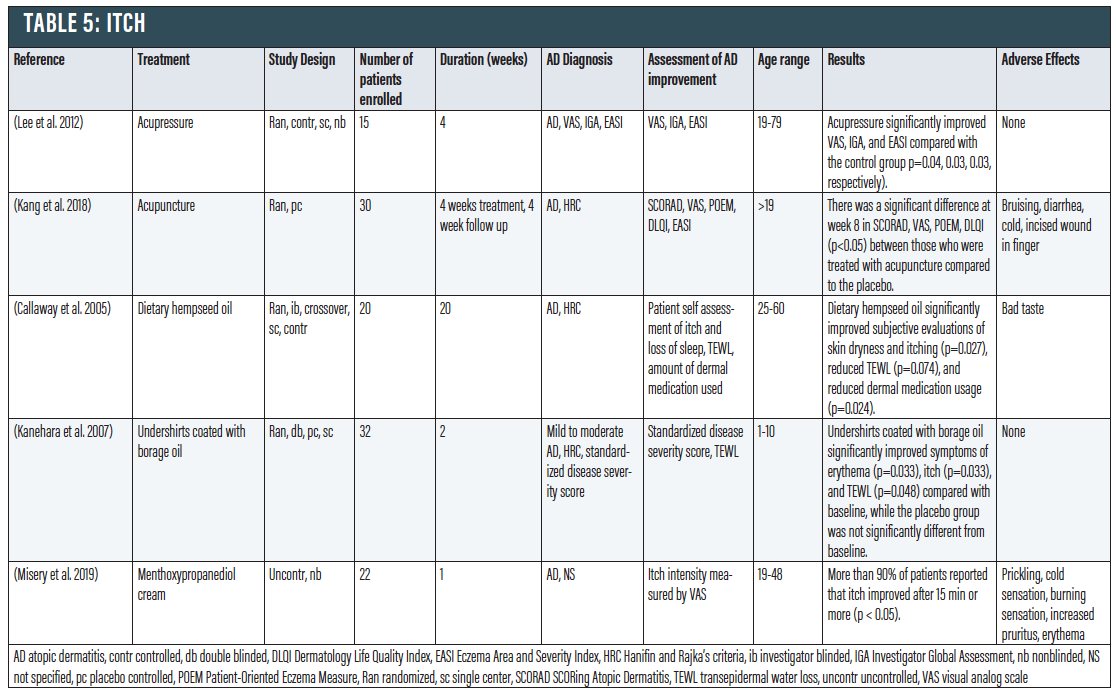
Acupuncture/Acupressure. Acupuncture has been shown to exhibit a significant effect on itch in contexts other than eczema. In a trial of 15 patients with moderate to severe AD, acupressure (using a small titanium bead to massage an acupoint on the arm three times weekly) significantly improved pruritus and lichenification compared with control group (p<0.04).106 Another study with 30 adults showed that four weeks of acupuncture led to a statistically significant improvement in SCORAD and itching intensity (p < 0.05) compared to sham-acupuncture.107
Dietary Hempseed Oil. Hempseed oil is a rich source of omega-6 and omega-3 polyunsaturated fatty acids, both of which have anti-inflammatory properties.108,109 In a study with 20 AD patients, daily consumption of two tablespoons of hempseed oil significantly improved subjective evaluations of itching, reduced TEWL (p=0.074), and reduced dermal medication usage compared to an olive oil control.110
Borage Oil-coated T-shirts. Borage oil has gamma-linolenic acid, an abundant lipid in the skin barrier.111 A clinical trial which had 16 children wore borage oil-coated t-shirts for two weeks demonstrated decreased symptoms of erythema and itch, and TEWL was also improved (p=0.048), while 16 children wearing placebo undershirts demonstrated no such improvement during the study period.111
Menthol. Cooling the skin by application of ice, gel packs, cool compresses, or cold water can temporarily reduce itch in patients affected by AD.112 In clinical experimental studies, cooling reduced itch and skin erythema caused by injection of histamine into the skin of healthy volunteers.113 Menthol was shown to alleviate itch in the same conditions in which cooling was effective, including AD, and in experimental studies inducing itch with injections of histamine or hydroxyethyl starch. Menthoxypropanediol cream is a synthetic derivative of menthol often used in cosmetics. A study showed that menthoxypropanediol cream was effective against itch in patients with AD, with a rapid effect, with more than 90 percent of patients reporting that itch improved after 15 minutes (p < 0.05).114
Conclusion
AD remains a complex and challenging disease, and it manifests differently in each patient. While conventional treatments do offer relief for many patients, the risks from such therapies coupled with the desire to personalize the approach may lead to the exploration of alternative and complementary treatments, working toward an integrative approach. These therapies remain an attractive adjuvant and occasional replacement for medications. This “toolbox” approach to AD management does not rely on a single treatment modality, but encourages those treating AD to explore a variety of therapies to offer relief.
No sources of funding were used to prepare this review. Helena Ma reports no conflicts of interest directly relevant to the content of this review. Dr. Shi is a consultant for Altus Labs. Dr. Lio reports research grants/funding from the National Eczema Association, Regeneron/Sanofi Genzyme, and AbbVie; is on the speaker’s bureau for Regeneron/Sanofi Genzyme, Pfizer, and L’Oreal; reports consulting/advisory boards for UCB, Dermavant, Regeneron/Sanofi Genzyme, Dermira, Pfizer, LEO Pharmaceuticals, AbbVie, Kiniksa, Eli Lilly, Micreos (stock options), La Roche Posay/L’Oreal, Pierre-Fabre, Johnson & Johnson, Level Ex, Unilever, Menlo Therapeutics, Theraplex, IntraDerm, Exeltis, AOBiome, Realm Therapeutics, Franklin Bioscience/Altus Labs (stock options), Verrica, TopMD, Arbonne, Amyris, Bodewell, and Burt’s Bees. In addition, Dr. Lio has a patent pending for a Theraplex product with royalties paid and is a Board member and Scientific Advisory Committee Member of the National Eczema Association.
1. Elmariah SB (2017) Adjunctive Management of Itch in Atopic Dermatitis. Dermatol Clin 35:373–394.
2. Waldman AR, Ahluwalia J, Udkoff J, Borok JF, Eichenfield LF (2018) Atopic Dermatitis. Pediatr Rev 39:180–193.
3. Wilkin JK, DuComb D, Castrow FF (1982) Scarification treatment of granuloma annulare. Arch Dermatol 118:68–69.
4. Malik K, Heitmiller KD, Czarnowicki T (2017) An Update on the Pathophysiology of Atopic Dermatitis. Dermatol Clin 35:317–326.
5. Vieira BL, Lim NR, Lohman ME, Lio PA (2016) Complementary and Alternative Medicine for Atopic Dermatitis: An Evidence-Based Review. Am J Clin Dermatol 17:557–581.
6. Levin J, Friedlander SF, Del Rosso JQ (2013) Atopic dermatitis and the stratum corneum: part 1: the role of filaggrin in the stratum corneum barrier and atopic skin. J Clin Aesthet Dermatol 6:16.
7. Danby SG, AlEnezi T, Sultan A, et al. (2013) Effect of olive and sunflower seed oil on the adult skin barrier: implications for neonatal skin care. Pediatr Dermatol 30:42–50.
8. Msika P, De Belilovsky C, Piccardi N, et al (2008) New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol 25:606–612.
9. Eichenfield LF, McCollum A, Msika P (2009) The benefits of sunflower oleodistillate (SOD) in pediatric dermatology. Pediatr Dermatol 26:669–675.
10. Nevin KG, Rajamohan T (2009) Wet and dry extraction of coconut oil: impact on lipid metabolic and antioxidant status in cholesterol coadministered rats. Can J Physiol Pharmacol 87:610–616.
11. Marina AM, Man YBC, Nazimah SAH, Amin I (2009) Antioxidant capacity and phenolic acids of virgin coconut oil. Int J Food Sci Nutr 60 Suppl 2:114–123.
12. Evangelista MTP, Abad-Casintahan F, Lopez-Villafuerte L (2014) The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, double-blind, clinical trial. International Journal of Dermatology 53:100–108.
13. Kurtz ES, Wallo W (2007) Colloidal oatmeal: history, chemistry and clinical properties. J Drugs Dermatol 6:167–170.
14. Food and Drug Administration, HHS (2003) Skin protectant drug products for over-the-counter human use; final monograph. Final rule. Fed Regist 68:33362–33381.
15. Saeed SA, Butt NM, McDonald-Gibson WJ, Collier HOJ (1981) Inhibitors of prostaglandin biosynthesis in extracts of oat (Aveena sativa) seeds. Biochem Soc Trans 9:144.
16. Sompayrac LM, Ross C (1959) Colloidal oatmeal in atopic dermatitis of the young. J Fla Med Assoc 45:1411–1412.
17. pp AB67–AB67
18. Lisante TA, Nuñez C, Zhang P (2017b) Efficacy and safety of an over-the-counter 1% colloidal oatmeal cream in the management of mild to moderate atopic dermatitis in children: a double-blind, randomized, active-controlled study. Journal of Dermatological Treatment 28:659–667.
19. Lisante TA, Nunez C, Zhang P, Mathes BM (2017a) A 1% Colloidal Oatmeal Cream Alone is Effective in Reducing Symptoms of Mild to Moderate Atopic Dermatitis: Results from Two Clinical Studies. J Drugs Dermatol 16:671–676.
20. Choe C, Schleusener J, Lademann J, Darvin ME (2017) In vivo confocal Raman microscopic determination of depth profiles of the stratum corneum lipid organization influenced by application of various oils. J Dermatol Sci 87:183–191.
21. Schwarzenberger K, Bergman JN, Chamlin SL (2014) Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies.
22. Matz H, Orion E, Wolf R (2003) Balneotherapy in dermatology. Dermatol Ther 16:132–140.
23. Proksch E, Nissen H-P, Bremgartner M, Urquhart C (2005) Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. International Journal of Dermatology 44:151–157.
24. Adler-Cohen C, Czarnowicki T, Dreiher J, Ruzicka T, Ingber A, Harari M (2012) Climatotherapy at the Dead Sea: an effective treatment modality for atopic dermatitis with significant positive impact on quality of life. Dermatitis 23:75–80.
25. Heinlin J, Schiffner-Rohe J, Schiffner R, et al (2011) A first prospective randomized controlled trial on the efficacy and safety of synchronous balneophototherapy vs. narrow-band UVB monotherapy for atopic dermatitis. JEADV 25:765–773.
26. Lee J, Lee SJ, Kim DS, Bang D (2007) The effect of wet-wrap dressing on epidermal barrier in patients with atopic dermatitis. J Eur Acad Dermatol Venereol 21:1360–1368.
27. Oranje AP, Devillers ACA, Kunz B, et al. (2006) Treatment of patients with atopic dermatitis using wet-wrap dressings with diluted steroids and/or emollients. An expert panel’s opinion and review of the literature. JEADV
28. Devillers ACA, Oranje AP (2006) Efficacy and safety of “wet-wrap” dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. British Journal of Dermatology 154:579–585
29. Schnopp C, Holtmann C, Stock S, Remling R, Fölster-Holst R, Ring J, Abeck D (2002) Topical steroids under wet-wrap dressings in atopic dermatitis--a vehicle-controlled trial. Dermatology 204:56–59.
30. Abeck D, Brockow K, Mempel M, Fesq H, Ring J (1999) Treatment of acute exacerbated atopic eczema with emollient-antiseptic preparations using the “wet wrap” (“wet pajama”) technique. Hautarzt 50:418–421.
31. Dennin M, Lio PA (2017) Filaggrin and childhood eczema. Arch Dis Child 102:1101–1102.
32. Tan SP, Brown SB, Griffiths C, Weller R, Gibbs NK (2017) Feeding filaggrin: effects of L-histidine supplementation in atopic dermatitis. Clinical, Cosmetic and Investigational Dermatology 10:403–411.
33. Bath-Hextall F, Delamere FM, Williams HC (2009) Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy 64:258–264.
34. Lopetuso LR, Scaldaferri F, Bruno G, et al (2015) The therapeutic management of gut barrier leaking: the emerging role for mucosal barrier protectors. Eur Rev Med Pharmacol Sci 19:1068–1076.
35. Gelmetti C (2000) Diet and atopic dermatitis. J Eur Acad Dermatol Venereol 14:439–440.
36. Pike MG, Heddle RJ, Boulton P, et al (1986) Increased Intestinal Permeability in Atopic Eczema. JID 86:101–104.
37. Souza SMC, Aquino LCM, Milach AC Jr, et al (2007) Antiinflammatory and antiulcer properties of tannins from Myracrodruon urundeuva Allemão (Anacardiaceae) in Rodents. Phytotherapy Research 21:220–225.
38. Shenefelt PD (2003) Biofeedback, cognitive-behavioral methods, and hypnosis in dermatology: is it all in your mind? Dermatol Ther 16:114–122.
39. Schachner L, Field T, Hernandez-Reif M, Duarte AM, Krasnegor J (1998) Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol 15:390–395.
40. Bae BG, Oh SH, Park CO, Noh S, Noh JY, Kim KR, Lee KH (2012) Progressive muscle relaxation therapy for atopic dermatitis: objective assessment of efficacy. Acta Derm Venereol 92:57–61.
41. Melin L, Frederiksen T, Noren P, Swebilius BG (1986) Behavioural treatment of scratching in patients with atopic dermatitis. Br J Dermatol 115:467–474.
42. Weber MB, Blessmann Weber M, da Luz Fontes Neto P de T, et al (2008) Improvement of pruritus and quality of life of children with atopic dermatitis and their families after joining support groups. JEADV 22:992–997.
43. Slyshenkov VS, Dymkowska D, Wojtczak L (2004) Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett 569:169–172.
44. Udompataikul M, Limpa-o-vart D (2012) Comparative trial of 5% dexpanthenol in water-in-oil formulation with 1% hydrocortisone ointment in the treatment of childhood atopic dermatitis: a pilot study. J Drugs Dermatol 11:366–374.
45. Stucker M, Pieck C, Stoerb C, et al (2004) Topical vitamin B12-a new therapeutic approach in atopic dermatitis-evaluation of efficacy and tolerability in a randomized placebo-controlled multicentre clinical trial. B J Dermatol 150:977–983.
46. Januchowski R (2009) Evaluation of Topical Vitamin B12 for the Treatment of Childhood Eczema. The Journal of Alternative and Complementary Medicine 15:387–389.
47. Nistico SP, Del Duca E, Tamburi F, et al (2017) Superiority of a vitamin B 12-barrier cream compared with standard glycerol-petrolatum-based emollient cream in the treatment of atopic dermatitis: A randomized, left-to-right comparative trial. Dermatol Ther 30:e12523.
48. Choi YK, Rho YK, Yoo KH, Lim YY, Li K, Kim BJ, Seo SJ, Kim MN, Hong CK, Kim D-S (2010) Effects of vitamin C vs. multivitamin on melanogenesis: comparative studyin vitroandin vivo. International Journal of Dermatology 49:218–226.
49. Sivaranjani N, Rao SV, Rajeev G (2013) Role of reactive oxygen species and antioxidants in atopic dermatitis. J Clin Diagn Res 7:2683–2685.
50. Lee JH, Jeon Y-J, Choi JH, Kim HY, Kim T-Y (2017) Effects of VitabridC12 on Skin Inflammation. Ann Dermatol 29:548–558.
51. Feily A, Namazi MR (2010) Vitamin A D Ointment Is Not an Appropriate Emollient for Atopic Dermatitis. Dermatitis 21:174–175.
52. Sharma S (2017) Correlation of Vitamin D3 Levels and SCORAD Index in Atopic Dermatits: A Case Control Study. J Clin Diagnostic Research
53. Borzutzky A, Camargo CA (2013) Role of vitamin D in the pathogenesis and treatment of atopic dermatitis. Expert Review of Clinical Immunology 9:751–760.
54. Camargo CA, Ganmaa D, Sidbury R, Erdenedelger K, Radnaakhand N, Khandsuren B (2014) Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. Journal of Allergy and Clinical Immunology 134:831–835.e1
55. Wiesenauer M (1984) Die Behandlung pruriginöser Dermatitiden mit Cardiospermum. Therapiewoche 34:5089–5091.
56. Ammon HPT, Kaul R (1992) Kamille: pharmakologie der kamille und ihrer inhaltsstoffe.
57. Patzelt-Wenczler R, Ponce-Pöschl E (2000) Proof of efficacy of Kamillosan (R) cream in atopic eczema. Eur J Med Res 5:171–175.
58. Uehara M, Sugiura H, Sakurai K (2001) A trial of oolong tea in the management of recalcitrant atopic dermatitis. Arch Dermatol 137:42–43.
59. Witte M, Krause L, Zillikens D, Shimanovich I (2019) Black tea dressings – a rapidly effective treatment for facial dermatitis. Journal of Dermatological Treatment 30:785–789.
60. Witte M, Krause L, Zillikens D, Shimanovich I (2019) Black tea dressings – a rapidly effective treatment for facial dermatitis. Journal of Dermatological Treatment 30:785–789.
61. Zink A, Traidl-Hoffmann C (2015) Green tea in dermatology - myths and facts. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 13:768–775.
62. Kim HK, Chang HK, Baek SY, et al (2012) Treatment of Atopic Dermatitis Associated with Malassezia sympodialis by Green Tea Extracts Bath Therapy: A Pilot Study. Mycobiology 40:124–128.
63. Marks DH, Friedman A (2018) The Therapeutic Potential of Cannabinoids in Dermatology. Skin Therapy Lett 23:1–5.
64. Pulvirenti N, Nasca MR, Micali G (2007) Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: a pilot study. Acta Dermatovenerol Croat 15:80–83.
65. Eberlein B, Eicke C, Reinhardt H-W, Ring J (2008) Adjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J Eur Acad Dermatol Venereol 22:73–82.
66. Walker D, Jacobe H (2011) Phototherapy in the age of biologics. Semin Cutan Med Surg 30:190–198.
67. Rodenbeck DL, Silverberg JI, Silverberg NB (2016) Phototherapy for atopic dermatitis. Clin Dermatol 34:607–613.
68. Grundmann SA, Beissert S (2012) Modern aspects of phototherapy for atopic dermatitis. J Allergy 2012:121797.
69. Bogh MKB, Gullstrand J, Svensson A, Ljunggren B, Dorkhan M (2012) Narrowband ultraviolet B three times per week is more effective in treating vitamin D deficiency than 1600 IU oral vitamin D3 per day: a randomized clinical trial. Br J Dermatol 167:625–630.
70. Dayal S, Pathak K, Sahu P, Jain VK (2017) Narrowband UV-B phototherapy in childhood atopic dermatitis: efficacy and safety. An Bras Dermatol 92:801–806.
71. Clayton TH, Clark SM, Turner D, Goulden V (2007) The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol 32:28–33.
72. Vaughn AR, Clark AK, Sivamani RK, Shi VY (2018) Circadian rhythm in atopic dermatitis-Pathophysiology and implications for chronotherapy. Pediatr Dermatol 35:152–157.
73. Kaur G, Phillips C, Wong K, Saini B (2013) Timing is important in medication administration: a timely review of chronotherapy research. Int J Clin Pharm 35:344–358.
74. Gupta MA, Gupta AK (2013) Sleep-wake disorders and dermatology. Clin Dermatol 31:118–126.
75. Kraft M (1999) Corticosteroids and leukotrienes: chronobiology and chronotherapy. Chronobiol Int 16:683–693.
76. Chang Y-S, Chou Y-T, Lee J-H, Lee P-L, Dai Y-S, Sun C, Lin Y-T, Wang L-C, Yu H-H, Yang Y-H, Chen C-A, Wan K-S, Chiang B-L (2014) Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics 134:e397–405.
77. Turek FW, Gillette MU (2004) Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists. Sleep Med 5:523–532.
78. Muñoz-Hoyos A, Espín-Quirantes C, Molina-Carballo A, et al (2007) Neuroendocrine and circadian aspects (melatonin and Β-endorphin) of atopic dermatitis in the child. Pediatr Allergy Immunol 18:679–686.
79. Chang Y-S, Chou Y-T, Lee J-H,et al. (2014) Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics 134:e397–405.
80. Chang Y-S, Lin M-H, Lee J-H, et al (2016) Melatonin Supplementation for Children With Atopic Dermatitis and Sleep Disturbance: A Randomized Clinical Trial. JAMA Pediatr 170:35–42.
81. Ardakani AT, Farrehi M, Sharif MR, et al (2018) The effects of melatonin administration on disease severity and sleep quality in children with atopic dermatitis: A randomized, double‐blinded, placebo‐controlled trial. Pediatric Allergy and Immunology 29:834–840.
82. Lio PA, Lee M, LeBovidge J, Timmons KG, Schneider L (2014) Clinical management of atopic dermatitis: practical highlights and updates from the atopic dermatitis practice parameter 2012. J Allergy Clin Immunol Pract 2:361–9
83. Nakatsuji T, Chen TH, Narala S, et al (2017) Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Science Translational Medicine 9:eaah4680.
84. Maarouf M, Shi VY (2018) Bleach for Atopic Dermatitis: Beyond Antimicrobials. Dermatitis. doi: 10.1097/DER.0000000000000358
85. Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS (2009) Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 123:e808–14.
86. Leung TH, Zhang LF, Wang J, Ning S, Knox SJ, Kim SK (2013) Topical hypochlorite ameliorates NF-κB-mediated skin diseases in mice. J Clin Invest 123:5361–5370.
87. Perez-Nazario N, Yoshida T, Fridy S, De Benedetto A, Beck LA (2015) Bleach baths significantly reduce itch and severity of atopic dermatitis with no significant change in S. aureus colonization and only modest effects on skin barrier function. In: Journal of Investigative Dermatology, pp S37–S37
88. Hon KL, Tsang YCK, Lee VWY,et al (2016) Efficacy of sodium hypochlorite (bleach) baths to reduce Staphylococcus aureus colonization in childhood onset moderate-to-severe eczema: A randomized, placebo-controlled cross-over trial. J Dermatolog Treat 27:156–162.
89. Gonzalez ME, Schaffer JV, Orlow SJ, Gao Z, Li H, Alekseyenko AV, Blaser MJ (2016) Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J Am Acad Dermatol 75:481–493.e8.
90. Lim NR, Treister AD, Tesic V, Lee KC, Lio PA (2019) A split body trial comparing dilute bleach vs. dilute apple cider vinegar compresses for atopic dermatitis in Chicago: a pilot study. J Dermat Cosmetol 3:22–24.
91. Chopra R, Vakharia PP, Sacotte R, Silverberg JI (2017) Efficacy of bleach baths in reducing severity of atopic dermatitis: A systematic review and meta-analysis. Ann Allergy Asthma Immunol 119:435–440.
92. Sawada Y, Tong Y, Barangi M, Hata T, Williams MR, Nakatsuji T, Gallo RL (2019) Dilute bleach baths used for treatment of atopic dermatitis are not antimicrobial in vitro. J Allergy Clin Immunol 143:1946–1948.
93. Verallo-Rowell VM, Dillague KM, Syah-Tjundawan BS (2008) Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis. Dermatitis 19:308–315.
94. Thormar H, Hilmarsson H (2007) The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem Phys Lipids 150:1–11.
95. Ryan KJ, Ray CG (2004) Medical microbiology. McGraw Hill 4:370.
96. Koller DY, Halmerbauer G, Böck A, Engstler G (2007) Action of a silk fabric treated with AEGISTMin children with atopic dermatitis: A 3-month trial. Pediatric Allergy and Immunology 18:335–338.
97. Hung M-H, Sartika D, Chang S-J, Chen S-J, Wang C-C, Hung Y-J, Cherng J-H, Chiu Y-K (2019) Influence of silk clothing therapy in patients with atopic dermatitis. Dermatol Reports 11:8176.
98. Gauger A, Mempel M, Schekatz A, Schäfer T, Ring J, Abeck D (2003) Silver-coated textiles reduce Staphylococcus aureus colonization in patients with atopic eczema. Dermatology 207:15–21.
99. Gauger A, Fischer S, Mempel M, Schaefer T, Foelster-Holst R, Abeck D, Ring J (2006) Efficacy and functionality of silver-coated textiles in patients with atopic eczema. Journal of the European Academy of Dermatology and Venereology 20:534–541.
100. Juenger M, Ladwig A, Staecker S, Arnold A, Kramer A, Daeschlein G, Panzig E, Haase H, Heising S (2006) Efficacy and safety of silver textile in the treatment of atopic dermatitis (AD). Curr Med Res Opin 22:739–750.
101. Araújo CP, Gomes J, Vieira AP, Ventura F, Fernandes JC, Brito C (2013) A proposal for the use of new silver-seaweed-cotton fibers in the treatment of atopic dermatitis. Cutan Ocul Toxicol 32:268–274.
102. Schmelcher M, Donovan DM, Loessner MJ (2012) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171.
103. Totté JEE, van Doorn MB, Pasmans SGMA (2017) Successful Treatment of Chronic Staphylococcus aureus-Related Dermatoses with the Topical Endolysin Staphefekt SA.100: A Report of 3 Cases. Case Rep Dermatol 9:19–25.
104. Romeo SP (1995) Atopic dermatitis: the itch that rashes. Pediatr Nurs 21:157–163.
105. Elmariah SB (2017) Adjunctive Management of Itch in Atopic Dermatitis. Dermatol Clin 35:373–394.
106. Lee KC, Keyes A, Hensley JR, G, et al (2012) Effectiveness of acupressure on pruritus and lichenification associated with atopic dermatitis: a pilot trial. Acupunct Med 30:8–11.
107. Kang S, Kim Y-K, Yeom M, et al (2018) Acupuncture improves symptoms in patients with mild-to-moderate atopic dermatitis: A randomized, sham-controlled preliminary trial. Complement Ther Med 41:90–98.
108. Simopoulos AP (2002) Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21:495–505.
109. Kapoor R, Huang Y-S (2006) Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol 7:531–534.
110. Callaway J, Schwab U, Harvima I, Halonen P, Mykkänen O, Hyvönen P, Järvinen T (2005) Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatolog Treat 16:87–94.
111. Kanehara S, Ohtani T, Uede K, Furukawa F (2007) Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: A double-blind, placebo-controlled clinical trial. The Journal of Dermatology 34:811–815.
112. Fruhstorfer H, Hermanns M, Latzke L (1986) The effects of thermal stimulation on clinical and experimental itch. Pain 24:259–269.
113. Bromm B, Scharein E, Darsow U, Ring J (1995) Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett 187:157–160.
114. Misery L, Santerre A, Batardière A, et al (2019) Real-life study of anti-itching effects of a cream containing menthoxypropanediol, a TRPM8 agonist, in atopic dermatitis patients. J Eur Acad Dermatol Venereol 33:e67–e69.
Recommended
- Atopic Dermatitis Journal Club
Journal Club: What's New in Pathogenesis and Treatment of Atopic Dermatitis










