CMS Updates Quality Reporting Requirements in Light of COVID-19

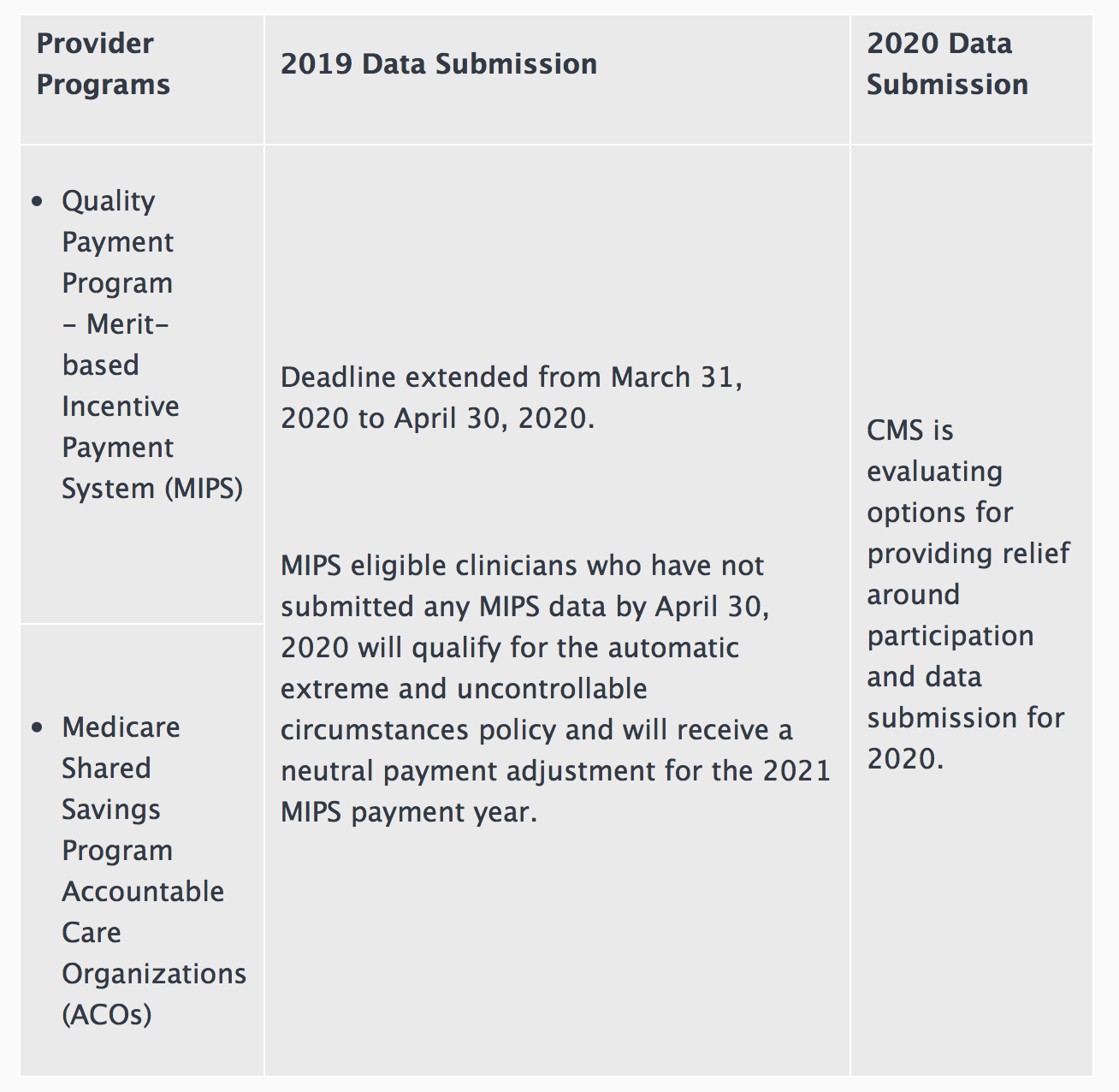
CMS is granting exceptions from reporting requirements and extensions for clinicians and providers participating in Medicare quality reporting programs with respect to upcoming measure reporting and data submission for those programs.
For those programs with data submission deadlines in April and May 2020, submission of those data will be optional, based on the facility’s choice to report. In addition, no data reflecting services provided January 1, 2020 through June 30, 2020 will be used in CMS’s calculations for the Medicare quality reporting and value-based purchasing programs. CMS says this is being done to reduce the data collection and reporting burden on providers responding to the COVID-19 pandemic.
CMS recognizes that quality measure data collection and reporting for services furnished during this time period may not be reflective of their true level of performance on measures such as cost, readmissions, and patient experience during this time of emergency and seeks to hold organizations harmless for not submitting data during this period.
 “In granting these exceptions and extensions, CMS is supporting clinicians fighting Coronavirus on the front lines,” says CMS Administrator Seema Verma in a statement. “The Trump Administration is cutting bureaucratic red tape so the healthcare delivery system can direct its time and resources toward caring for patients.”
“In granting these exceptions and extensions, CMS is supporting clinicians fighting Coronavirus on the front lines,” says CMS Administrator Seema Verma in a statement. “The Trump Administration is cutting bureaucratic red tape so the healthcare delivery system can direct its time and resources toward caring for patients.”
CMS says it will continue monitoring the developing COVID-19 situation and assess options to bring additional relief to clinicians, facilities, and their staff so they can focus on caring for patients.
A complete and updated list of CMS actions and other information specific to CMS, is available at the Current Emergencies Website.
