Practical Considerations in Pediatric Alopecia
A clinical update on the diagnosis and treatment of tinea capitis, alopecia areata, trauma secondary to traction or trichotillomania, and telogen effluvium in children.
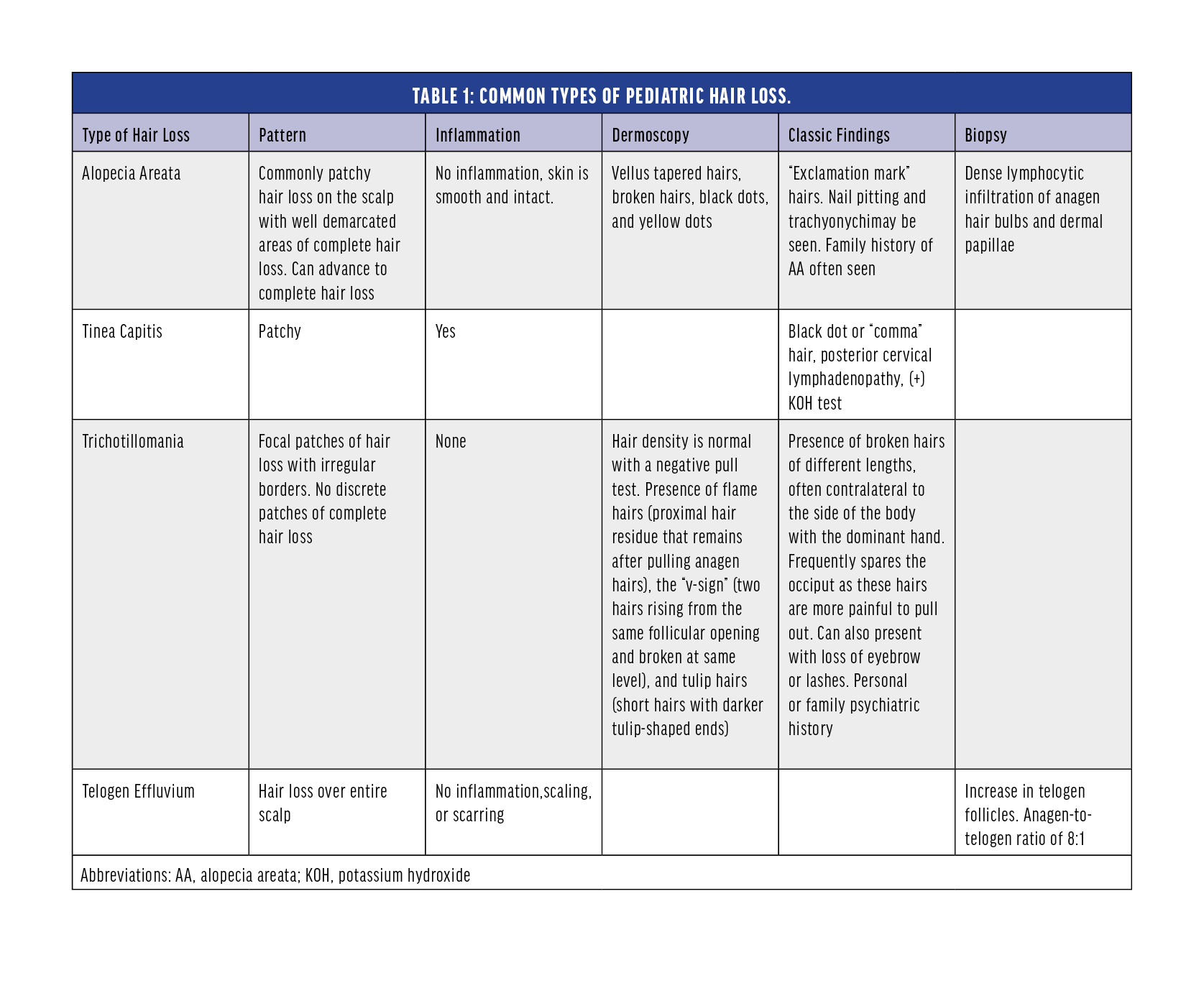
Hair loss in children encompasses a wide spectrum of conditions that may be congenital or acquired. Hair consists of the proteinaceous shaft and the root, anchored in the follicle, an involution of the epidermis.1 Newborn follicles produce soft lanugo hair, which is replaced by downy, fine vellus hair after 3 to 4 months, and then androgens transform most vellus hair into thicker terminal hairs at puberty.1 Terminal hairs go through cyclical growth with up to 90% of follicles in active growth (anagen phase), 1-3% in a brief transition (catagen phase), and 5-10% in dormancy (telogen phase). Hair is shed after 2 to 3 months in the telogen phase, after which anagen begins again, and the cycle repeats.1 There are two main types of alopecia: those associated with hair follicle damage or destruction are referred to as scarring alopecias, whereas those where the follicle is preserved are considered nonscarring.2 While there are many specific diseases that can result in hair loss, the most common causes of pediatric alopecia are tinea capitis, alopecia areata, trauma secondary to traction or trichotillomania (TTM), and telogen effluvium (TE).3 In this brief review, we will focus on these common diagnoses with an emphasis on diagnosis and treatment options.
Tinea capitis (ringworm of the scalp) is a highly contagious dermatophyte fungal infection of the scalp and hair. The greatest culprit worldwide remains Microsporum canis which can be acquired from animals, whereas in the US and UK, it is Trichophyton tonsurans which typically spreads between human contacts.1 The dermatophytes invade the hair follicle, resulting in acute patchy hair loss with erythema, pruritus, and scaling.
Alopecia areata (AA) is a chronic immune-mediated disease that usually presents as acute or chronic patchy loss of hair, most often from the scalp. During acute flares, the anagen phase is significantly shortened, forcing the hair follicle into a premature catagen phase and eventually apoptosis. AA lesions show dense perifollicular and intrafollicular inflammatory cell infiltrate around the bulb consisting of CD8+ T and CD4+ T cells, mast cells, NK cells, and dendritic cells4. Important mediators leading to proinflammatory factors include interferon-gamma (IFN-γ), substance P, interleukin-2 (IL-2), and interleukin-15 (IL-15).5
Trichotillomania (TTM or trichotillosis) refers to repetitive, compulsive, and self-induced hair pulling that may fall into the spectrum of obsessive-compulsive disorders. Although it can occur in children under 6 years old, the peak incidence is age 9 to 13 years with a strong female predominance.6
Lastly, TE refers to an abnormality of the normal hair cycle that results from a disruption in the transition from the anagen to the telogen phase due to psychological stress, chronic illness, malnutrition, severe infection, metabolic disturbances, surgery, and medications.5 It is usually self-limiting as the alopecia will resolve as new anagen hair grows over 3 to 6 months.
The prevalence of tinea capitis in children is estimated to be 4-13%, the point prevalence of alopecia areata is approximately 1 in 1000 people with a lifetime risk of approximately 2%, and 2.7% of children present with acute TE.3 The prevalence of traction alopecia and TTM is not easily estimated because of underdiagnosis and secretive behaviors.
Clinical presentation of pediatric alopecia ranges from subtle to disfiguring and may lead to low self-esteem, depression, and social isolation. Therefore, proper management requires a holistic approach not only for the child patient but the parents and surrounding environment as well. Differentiation of alopecia due to benign causes from that due to serious illness is important for reducing patient and parent distress and offering adequate and prompt diagnosis and treatment.
ASSESSMENT AND DIAGNOSIS
Patient History: Taking a systematic, thorough history is essential for diagnosis, ranging from questions about the nature of hair loss to even broader questions regarding stressors in life. A thorough history will ideally include5:
- Whether there is visible hairlessness (alopecia) or an increased amount of hair falling out daily (TE)
- Age of onset
- Distribution, such as focal, patterned, or diffuse
- Associated symptoms
- Family history
- Medications and dietary changes
Physical Exam: Physical assessment of a child with alopecia involves a complete skin examination. Assess for evidence of erythema, scales, pustules or papules, erosions, and excoriation, which may all be associated with alopecia or signs of an associated scalp disorder.3 A closer inspection involves using a dermoscopy for visualizing epidermal and subepidermal structures undetectable by the naked eye.5 Furthermore, the hair pull test identifies active hair shedding and should be performed on all patients presenting with hair loss. Approximately 50 hairs are grasped at the skin surface and consistent pressure is applied from the proximal to distal ends. The easy extraction of more than three hair fibers per 60 hairs suggests increased hair shedding and fragility.5 Extracted hair should be visualized microscopically to determine the phase (anagen or telogen) and whether the hair is broken or dystrophic.1
Laboratory and Other Testing:
Ordering laboratory tests can shed light on the underlying causes of hair loss. The following basic investigation parameters could be considered for patients with diffuse hair loss of uncertain etiology7:
- Complete blood count (CBC) and complete metabolic panel (CMP)
- Serum vitamin D
- Thyroid function tests
- Iron studies
- ANA (Antinuclear Antibody) test
Particularly for patients with AA, the Severity of Alopecia Tool (SALT) can help guide therapeutic decision-making and monitor the therapeutic response. The scalp area is divided into 4 quadrants, with each quadrant representing a percentage of the total scalp area: right side (18%), left side (18%), top (40%), and back (24%).5 The percentage of hair loss is visually estimated in each quadrant and then summated to determine the SALT score, with the 5 subgroups of hair involvement as follows: S0 = no hair loss, S1 = less than 25% hair loss, S2 = 25 to 49% hair loss, S3 = 50 to 74% hair loss, S4 = 75 to 99% hair loss, and S5 = 100% hair loss.5 (See Table 1.)
CURRENT MANAGEMENT FOR PEDIATRIC HAIR LOSS
Trichotillomania (TTM) in preschool-aged children is considered a habit disorder and has a benign course, generally disappearing by school age, and can often be managed conservatively with gentle reminders1. In older patients, TTM can present with other psychiatric comorbidities and may benefit from a psychiatric evaluation and behavioral therapy. Of note is habit reversal therapy (HRT), which involves targeting the patient's pulling behaviors through awareness training, self-monitoring, stimulus identification and elimination, and competing response procedures that produce behaviors incompatible with pulling when pulling urges occur8. Behavioral therapy like HRT emphasizes the importance of the child patient, patient, and physician all learning to recognize the source of the issue holistically and working together to closely monitor and promptly refine the patient's individualized treatment plan to enhance therapeutic effectiveness through a multidisciplinary approach.
Telogen effluvium is generally reversible, with trigger removal and treating the underlying disorder if one is present.
Tinea capitis can be treated with oral antifungal medications in addition to antifungal shampoos, and some children under 1 year may be treated with topical azoles alone.1
Compared to the other types of hair loss, alopecia areata lacks a consistently effective treatment, with even fewer therapeutic options for children. Current therapies aim to modulate the activity of the disease, with generally unsatisfying responses and high relapse rates.4
Topical Steroids: Topical corticosteroids are recommended as a first-line therapy for treating limited patchy alopecia areata and adjunctive therapy in more severe forms because of their relatively favorable side-effect profile and their ability to be used in children younger than 10 years of age. Diverse topical formulations of corticosteroids are available, such as solutions, shampoos, oils, or foam preparations. Adverse effects include folliculitis and rarely, skin atrophy, which in most cases is reversible when identified early.4 Their key limitations are the difficulty in wider application and their modest efficacy
Intralesional Injection of Corticosteroids: Injections are useful mainly for limited patchy AA. In responders, hair regrowth is expected to be seen in about 4-8 weeks. The most common side effect is mild pain during injection and the development of skin atrophy.4
Systemic Corticosteroids: The therapeutic response to systemic corticosteroids varies greatly between studies, ranging from 28-84% efficacy, and relapse rates were reported between 25-75%.4 Furthermore, though few serious or long-term side effects have been reported in children in this context, the side effects of long-term systemic steroid use are well characterized and present a serious obstacle for wider use in AA.
Topical Sensitization/Irritation Therapy: Topical sensitization with diphenylcyclopropenone (DPCP) is recommended for chronic (>2 years) severe AA in children >12 years old [5]. DPCP induces allergic contact dermatitis that enables hair regrowth through an unclear mechanism of immunomodulation involving antigenic competition.4 Treatment response is expected after approximately 4 months of treatment and is then continued until maximum response is achieved, followed by maintenance therapy with longer spaced intervals. However, clinical studies show poor response and high relapse rates in children, and there is potential for unfavorable adverse effects including occipital or cervical lymphadenopathy, severe dermatitis, and flu-like symptoms.4Anthralin is an alternative to DPCP used in extensive AA especially in children because of its fewer side effects and the advantage of it being able to be used at home. However, only a few studies on the efficacy of anthralin as monotherapy exist, and response rates vary greatly, from 18-75%.4
AA management requires a personalized tailored approach to each patient’s specific AA characteristics, including the extent and location of scalp-hair loss, duration of AA, and psychosocial impact of disease.9 Robust treatment efficacy data for AA are still limited and patients with AA are often dissatisfied with traditional treatments. Furthermore, relapse rates remain high especially among patients with extensive AA; therefore, maintenance therapy is usually necessary which is problematic because many therapies for extensive AA have adverse effect profiles and limit long-term use.9 The greatest unmet needs in the treatment of AA are long-term disease control, improved efficacy profiles, faster onset of action, and better safety profiles.10
JAK INHIBITORS
New insights into the pathophysiology of AA show that Interferon gamma (IFN-γ)-driven inflammation is JAK-STAT mediated and promotes the inflammatory feedback loop that further contributes to local inflammation.4 Normally, the anagen hair bulb is protected from the immune system and in a state of decreased self-antigen presentation that prevents T-cell activation. However, in AA, inflammatory cytokines like IL-2/IL-15 and CD8+ T-cells predominate and further activate IFN-γ, creating a positive feedback loop.11 Due to the crucial role of JAK-STAT pathway in AA pathogenesis, it is no surprise that JAK inhibitors (JAKis) are the new emerging therapeutic option.
In June 2022, baricitinib became the first US FDA-approved JAKi treatment for alopecia areata, but only in adults.12 Ritlecitinib is FDA-approved for individuals 12 years of age and older with severe alopecia areata. Other JAKis have also been used off-label to treat AA, such as the first-generation JAKis tofacitinib and ruxolitinib, and more selective second-generation JAKis including upadacitinib and jaktinib.11 There have been many case reports and case series reporting the benefits of JAKis for some AA patients, but studies having high levels of evidence are still lacking and the existing ones mainly focus on adult patients, which makes this class of medications a less feasible option for children.
The ALLEGRO 2b/3 is one of the few completed randomized clinical trials studying the safety and efficacy of JAKis (specifically ritlecitinib) that included pediatric patients 12 years of age and older as well as adult patients. The patients had severe AA (SALT score >50) and were randomized to receive 50 mg, 30 mg, 10 mg of ritlecitinib, or placebo. A greater proportion of patients who took ritlecitinib 30 mg or 50 mg met the primary endpoint of SALT score ≤ 20 after 24 weeks compared with placebo.11
A subgroup analysis specifically evaluating the efficacy and safety of ritlecitinib in patients aged 12-17 years from the ALLEGRO 2b/3 study showed that at week 24, higher proportions of adolescents in the ritlecitinib 30 mg or 50 mg had SALT score ≤ 20 and the more stringent endpoint of SALT score ≤ 10 versus placebo and ritlecitinib 10 mg.12 Overall, the mean change from baseline in SALT score demonstrated a dose-dependent effect and was greater in 30 mg and higher ritlectinib groups versus placebo through week 24. Higher rates of patient-reported improvement in AA and satisfaction with outcome were also observed with ritlecitinib versus placebo, and overall efficacy outcomes in adolescents were consistent with those in the total study population (adolescents and adults). Ritlecitinib was well tolerated at all doses and the safety profile suggests no clinically concerning long-term effects reported. Since this study, there have been more currently active phase 2 and 3 clinical trials studying ritlecitinib and other JAKis including ruxolitinib and tofacitinib in pediatric patients.11
However, most of the literature to date on JAKis for AA are case reports and case series in the pediatric population. (See Table 2.)

A meta-analysis by Chen et al. examined 69 pediatric patients from 10 studies, reporting JAKis were effective in 81.9% of patients, and 68.5% showed a decrease in SALT score or regrowth of more than 50%.13 Furthermore, similarly to adults, the use of oral JAKis is linked to a more effective response than topical medications. A meta-analysis of seven observational studies (59 total patients from ages 4-19) on tofacitinib in the pediatric population with moderate-severe AA suggests promising outcomes with good/complete response in 55% and at least a partial response in just above 40%, such that more than 95% of patients showed some response in the trial.14 Meta-analysis showed that oral administration was significantly more efficacious than topical application (73% vs 23%, P = 0.04).14 However, there are concerns regarding long-term oral therapy's adverse effects. Although most short-term adverse events were minor and reversible, some common adverse events in children taking JAKis orally were liver transaminase elevation (18.4%), upper respiratory tract infection (14.3%), and eosinophilia (10.2%).13 This calls for specific laboratory testing and close monitoring during treatment, which could be a barrier for some.
Studies on topical JAKis seem to suggest less efficacy than oral JAKis in treating AA. A study by Olsen et al. assessed the efficacy of 1.5% ruxolitinib in patients with AA and found no significant difference in hair regrowth after 48 weeks.15 However, there have been some case reports of success with topical JAKis. Craiglow et al. reported successful treatment of alopecia universalis with topical ruxolitinib 0.6% cream in a teenage girl.16 Other studies on topical JAKis also show some promising results with improvement in SALT score and a potentially more favorable safety profile than its oral counterpart.17 Overall, topical JAK inhibitors in children–even if they are found to be effective in some cases–are limited to the treatment of more localized diseases, as is the case for adult patients.
UNMET NEEDS AND INCREASING AWARENESS AND ADVOCACY
There is still a lack of data on longitudinal complications, tapering strategies, and optimal maintenance dosage regimens for JAKis. There is a need to further assess the long-term safety and efficacy of both oral and topical JAKis in children, as patients with AA have a higher rate of disease relapse with JAKis discontinuation and likely require continuation of the lowest effective dose longitudinally. Studies with higher levels of evidence and longer follow-up periods are therefore needed before more definitive conclusions can be made.
Improvement in the understanding of the pathogenesis of AA and the need for more effective and safer therapies is just one aspect of the unmet needs of AA. Lack of access to treatments and cost is another major issue of JAKis. Many insurances have restrictions regarding medication coverage for JAKis, requiring multiple appeals or off-label therapies.18 This is a sizable hurdle since JAKi discontinuation often results in recurrence, and patients generally need longitudinal maintenance therapy. More advocacy is necessary to recognize the substantial burden of AA and substantiate this autoimmune disease as deserving of intervention.
Another substantial issue is the psychological burden and quality of life in patients. A study investigating all-cause and cause-specific mortality in patients with AA found the mortality risk associated with self-harm and psychiatric diseases was significantly higher in AA patients compared to matched controls (HR, 1.21; 95% confidence interval (CI), 1.04–1.41).19 Furthermore, a recent study examining the impact of AA on quality of life and bullying in pediatric patients demonstrated a high prevalence of bullying and decreased engagement in social activities in this population, leading to higher rates of depression and anxiety.18
Addressing mental health comorbidities and issues with the quality of life is particularly important in pediatric patients with AA. Treatment should be a multi-disciplinary effort that incorporates measures of mental health and access to support groups and non-medical therapies as well. Support groups can help patients with AA develop coping strategies and learn various ways of navigating life. The National Alopecia Areata Foundation (NAAF) connects patients with AA to support groups all around the world, and online support networks also exist to increase accessibility for patients. However, attending a support group requires free time, transportation (if in-person), webcam, and an internet connection (if virtual), which raises the question on whether there are socioeconomic disparities in access to support groups.19
Cranial prostheses and camouflaging techniques such as wigs also improve quality of life. Despite evidence of their benefits, they also remain an underfunded tool by health insurance. A study analyzing the financial burden of AA demonstrated that spending was highest for camouflaging techniques including wigs and makeup, leaving patients with AA in lower socioeconomic status unable to adequately manage their disease.19
In conclusion, the optimal treatment course requires careful consideration by clinicians of the evidence and adverse effect profile of each treatment option, along with individual patients’ goals and shared decision-making. Greater awareness and education of alopecia from the community at large is also necessary to break down social stigma, improve acceptance of and inclusion for those with alopecia, and advocate for legislation as well as programs to support these patients.
1) Xu L, Liu KX, and Senna MM (2017) A Practical Approach to the Diagnosis and Management of Hair Loss in Children and Adolescents. Front. Med. 2017;4:112. doi:10.3389/fmed.2017.00112
2) eid EE, Lio PA. Chapter 23. Hair Loss. In: Henderson MC, Tierney LM, Jr., Smetana GW. eds. The Patient History: An Evidence-Based Approach to Differential Diagnosis. McGraw Hill; 2012. Accessed May 30, 2022. https://accessmedicine.mhmedical.com/content.aspx?bookid=500§ionid=41026568
3) Cranwell W, Sinclair R. Common causes of paediatric alopecia. Aust J Gen Pract. 2018;47(10):692-696. doi:10.31128/AJGP-11-17-4416
4) Lintzeri, D.A., Constantinou, A., Hillmann, K., Ghoreschi, K., Vogt, A., and Blume- Peytavi, U. (2022), Alopecia areata – Current understanding and management. JDDG: Journal der Deutschen Dermatologischen Gesellschaft, 2022;20:59-90. doi.org/10.1111/ddg.14689
5) Al Aboud AM, Zito PM. Alopecia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; Jan 2023.
6) Pereyra AD, Saadabadi A. Trichotillomania. In: StatPearls. Treasure Island (FL): StatPearls Publishing; June 26, 2023.
7) Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of Telogen Effluvium in India. Int J Trichology. 2019;11(3):107-112. doi:10.4103/ijt.ijt_23_19
8) Morris Sarah H, Zickgraf Hana F, Dingfelder Hilary E & Franklin Martin E. (2013) Habit reversal training in trichotillomania: guide for the clinician. Expert Review of Neurotherapeutics. 2013;13(9):1069-1077. doi:10.1586/14737175.2013.827477
9) Brett King, Edmund Pezalla, Selwyn Fung, Helen Tran, Jeffrey A Bourret, Kathleen Peeples-Lamirande, Liza Takiya, and Lynne Napatalung. Overview of alopecia areata for managed care and payer stakeholders in the United States. Journal of Managed Care & Specialty Pharmacy. 2023;29(7):848-856.
10) Ammoury, A., Hegazy, R., Al Talhab, S. et al. Treatment Patterns and Unmet Needs in the Management of Alopecia Areata: Results of a Physician’s Survey in the Middle East. Dermatol Ther (Heidelb). 2023;13:1801-1815. doi.org/10.1007/s13555-023-00963-7
11) Haughton R, Herbert SM, Ji-Xu A, Downing L, Raychaudhuri SP, Maverakis E. Janus kinase inhibitors for alopecia areata: A narrative review. Indian J Dermatol Venereol Leprol. 2023;doi: 10.25259/IJDVL_1093_2022
12) Hordinsky, M, Hebert, AA, Gooderham, M, et al. Efficacy and safety of ritlecitinib in adolescents with alopecia areata: Results from the ALLEGRO phase 2b/3 randomized, double-blind, placebo-controlled trial. Pediatr Dermatol. 2023;doi:10.1111/pde.15378
13) Yi Chen, Huijun Zhu, Yuqing Shen, Yuqi Zhu, Jiayi Sun, Yeqin Dai & Xiuzu Song (2022) Efficacy and safety of JAK inhibitors in the treatment of alopecia areata in children: a systematic review and meta-analysis. Journal of Dermatological Treatment; 33;8:3143-3149. doi: 10.1080/09546634.2022.2133956
14) Behrangi, E, Barough, MS, Khoramdad, M, Hejazi, P, Koltapeh, MP, Goodarzi, A. Efficacy and safety of tofacitinib for treatment of alopecia areata in children: A systematic review and meta-analysis. J Cosmet Dermatol. 2022;21:6644-6652. doi:10.1111/jocd.15425
15) Elise A. Olsen, Deanna Kornacki, Kang Sun, Maria K. Hordinsky. Ruxolitinib cream for the treatment of patients with alopecia areata: A 2-part, double-blind, randomized, vehicle-controlled phase 2 study. J Am Academ Dermatol. 2020;82(2):412-419. doi: 10.1016/j.jaad.2019.10.016
16) Craiglow BG, Tavares D, King BA. Topical Ruxolitinib for the Treatment of Alopecia Universalis. JAMA Dermatol. 2016;152(4):490–491. doi:10.1001/jamadermatol.2015.4445
17) Putterman E and Castelo-Soccio L. Topical 2% tofacitinib for children with alopecia areata, alopecia totalis, and alopecia universalis. J Am Academ Dermatol. 2018;78(6):1207-1209. doi:10.1016/j.jaad.2018.02.031
18) Kibbie, J, Kines, K, Norris, D, Dunnick, CA. Oral tofacitinib for the treatment of alopecia areata in pediatric patients. Pediatr Dermatol. 2022; 39: 31–34. doi:10.1111/pde.14855
19) Alhanshali L, Buontempo MG, Lo Sicco KI, Shapiro J. Alopecia Areata: Burden of Disease, Approach to Treatment, and Current Unmet Needs. Clin Cosmet Investig Dermatol. 2023;16:803-820. Published 2023 Mar 31. doi:10.2147/CCID.S376096
20) Hamilton CE and Craiglow BG. JAK Inhibitors for the Treatment of Pediatric Alopecia Areata. JISP. 2020;20(1):31-36. doi:10.1016/j.jisp.2020.04.005
21) Kołcz K, Żychowska M, Sawińska E, Reich A. Alopecia Universalis in an Adolescent Successfully Treated with Upadacitinib-A Case Report and Review of the Literature on the Use of JAK Inhibitors in Pediatric Alopecia Areata. Dermatol Ther (Heidelb). 2023;13(3):843-856. doi:10.1007/s13555-023-00889-0
22) Zhao J, Liu L. A case of atopic dermatitis with alopecia universalis in a patient treated with abrocitinib. JAAD Case Rep. 2022;22:99-100. doi:10.1016/j.jdcr.2022.02.027
