Management of Atopic Dermatitis During the COVID-19 Pandemic
In December 2019, a series of acute respiratory illnesses of unknown cause emerged from Wuhan, Hubei provence, in China, with clinical presentations greatly resembling viral pneumonia.1-3 A novel betacoronavirus was identified and subsequently named as the 2019 novel coronavirus (2019-nCoV or SARS-CoV-2).1-3

The COVID-19 pandemic has been a dramatic and unprecedented challenge for all healthcare systems globally.4 Due to the recent pandemic, there is concern about the immunosuppressive or immunomodulatory effects of oral and biologic medications in patients with skin disease, particularly atopic dermatitis and psoriasis. Currently, the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have no guidelines on the use of biologics during the COVID-19 pandemic.5 In addition, there is no data regarding the increased risk of infection or disease severity in patients exposed to COVID-19 who are receiving systemic treatment for atopic dermatitis (AD). In this article, we will address recommendations on the use of oral and biologic agents in AD patients during the COVID-19 outbreak.5
AD and COVID-19: recommendations FOR oral and biologic agent use in AD patients
Both CD8+ and CD4+ T-cell immunity are critical to host defense against viral pathogens. The best characterized of these CD4+ T-cells are the T helper 1 (Th1) and Th2 cells, which are characterized by their production of interferon-γ (IFN-γ) and interleukin-4 (IL-4), respectively.6 During a viral infection, native T cells recognize antigens on activated antigen-presenting cells (APCs), which results, predominantly, in the generation of Th1 cells due to the presence of type I interferons (IFNs) and IL-12. Importantly, Th17, Th2, and regulatory T (TReg) cell populations are also generated to some degree to combat certain viral infections.6 With the SARS-CoV-2 virus, the initial stages of infection are characterized by the expression of type I IFNs and other pro-inflammatory cytokines, as well as the activation of Th1/Th17 cells, which exacerbates host inflammatory responses.7 Given the importance of humoral immunity in detection and neutralization of viral pathogens, there is concern that immunocompromised or immunosuppressed patients are at a greater risk of infection and increased morbidity and mortality when infected with SARS-CoV-2 virus.8
Adults with atopic disease (asthma, allergic rhinitis, AD) are at a significantly increased risk of lower respiratory tract infections (LRTIs; including acute bronchitis and pneumonia) and upper respiratory tract infections (URTIs; including common cold, sinusitis, tonsillitis, and otitis media) compared to patients without atopic disease.9 It is known that atopic diseases share similar immunopathological profiles that predispose patients to respiratory infections. Nonetheless, since dermatitis is a more consistent manifestation of the atopic constitution, the reported association with respiratory infections may be an overestimate and instead more representative of the association between the general allergic phenotype and respiratory infections.9 A plethora of evidence indicates that AD increases susceptibility to bacterial, fungal, and viral skin infections.10 Patients with allergic rhinitis and asthma, however, exhibit more numerous, prolonged, and severe respiratory infections than nonallergic subjects.11,12 In addition, recurrent respiratory infections affecting the lower respiratory tract have been shown to be associated with asthma and allergic rhinitis in children.13 While patients with atopic profiles may experience a greater number of minor URTIs, the evidence supports that AD is not an independent factor that predisposes individuals for severe viral respiratory infections, such as SARS-CoV-2 infection. This is consistent with the fact that primary AD is an inflammatory skin disease characterized by immune dysregulation and an overreactive immune system and is only associated with a number of immunodeficiency syndromes with secondary disease.14
Patients with moderate to severe AD generally require treatment with oral or biologic agents, which exhibit immunosuppressive or immunomodulatory effects. The FDA-approved agent (dupilumab) and “off-label” drugs (cyclosporine, azathioprine, mycophenolate mofetil, methotrexate, and prednisone) for moderate to severe AD carry unique risk profiles and distinct recommendations for patients with active infection or signs and/or symptoms of infection (Table 1). These medication-specific recommendations should guide providers when treating AD patients with active infection or signs and/or symptoms of infection.

Unlike other biologics, dupilumab targets the activity of IL-4 and IL-13, cytokines important in type 2 immune pathways, but not crucial to host defense mechanisms against most infectious agents, except endoparasites.15 Currently, there are no recommendations in place for dupilumab treatment in the presence of non-helminth infections. In several randomized, placebo-controlled dupilumab trials in adults with moderate to severe AD, dupilumab did not increase overall infection risk and was associated with lower rates of serious or severe infections and lower rates of non-herpetic skin infections compared to the placebo.15 In two case reports from Milan, a geographic area with a high incidence of COVID-19 infection, only two (0.82 percent) patients, of 245 patients treated with dupilumab, developed COVID-19 infection during treatment.16 The patients with COVID-19 infection did not experience any complications during the course of their infection. Furthermore, dupilumab was shown to be an effective and safe therapy for patients with severe AD, even in cases of severe infections, such as COVID-19 infection.16 The European Task Force on Atopic Dermatitis recently declared that targeted treatment selectively interfering with type 2 inflammation (such as dupilumab) should not increase the risk of viral infections and thus, may be preferred over conventional systemic immuno-suppressive treatments, such as cyclosporine, during the COVID-19 pandemic.17 This theoretical advantage, however, is not supported by robust clinical data and the task force recommends that all doctors treating AD patients to remain vigilant and up-to-date on developing data.17 Altogether, the evidence suggests that AD patients treated with dupilumab can continue treatment safely despite having an infection, as long as the infection is non-serious, monitored, and uncomplicated, and the provider is informed of the infection (Table 1). Since SARS-CoV-2 is a novel virus that is highly contagious and can result in severe disease leading to hospitalization, admission to an intensive care unit, or death in both young healthy and elderly patients, COVID-19 is thought to be a serious disease with high morbidity and mortality.18 AD patients diagnosed with COVID-19 or with signs and/or symptoms of COVID-19 should, therefore, discontinue dupilumab treatment given that COVID-19 is a serious infection and clinical data regarding dupilumab’s potential advantage is limited (Table 1).
Risk-assessment in AD patients with significant comorbidities
With the recent COVID-19 pandemic, there has been concern about the safety of biologic and oral agents in patients with AD, particularly those with high-risk comorbidities.19 AD patients < 60 years old, without comorbidities, and without evidence or signs and symptoms of COVID-19 infection are not required to discontinue oral and/or biologic treatment or delay treatment initiation.5,20 The possible exception to these recommendations is systemic corticosteroid therapy, which needs to be weaned instead of immediately discontinued in AD patients.21 Elderly patients are at a higher risk of severe complications due to age-related decreases in humoral immunity.22,23 An intrinsic defect in the ability of B cells to undergo class switching exists in these patients, leading to reduced quantities of IgA, IgE, and IgG produced by activated B cells.22 Aging also leads to decreased production of fresh naïve T cells, limited T-cell receptor behavior, and weakened activation of T cells.23 This is evidenced by the finding that the mortality rate of patients with COVID-19 infection who are aged > 60 years is significantly higher (5.3 percent) than that of patients under 60 years (1.4 percent).24
These recommendations are in line with summary guidelines from the American Academy of Dermatology (AAD)-National Psoriasis Foundation (NPF) for managing active infection in psoriasis patients that are receiving biologics.25 AD is associated with a number of immunodeficiency syndromes, but is not believed to be a predisposing factor for a weaker immune system; rather, it is characterized by immune dysregulation or an over-active immune state with Th2 polarization.26-28 In patients without COVID-19 infection, physicians should weigh the risks and benefits of oral and biologic treatment for AD on a case-by-case basis, with consideration of the following factors:5,19
- original indication of the oral or biologic agent
- severity of the original indication
- the patient’s age
- presence of comorbidities.
Certain pre-existing comorbidities put patients at increased risk of severe disease and mortality with COVID-19 infection. High-risk comorbidities associated with severe disease and mortality with COVID-19 infection include:19,29
- hypertension
- respiratory disease (COPD, asthma)
- diabetes
- cancer
- kidney disease
- cardiovascular disease
- immunocompromised states
AD patients > 60 years old or with high-risk comorbidities are required to discontinue oral and/or biologic treatment or delay treatment initiation, regardless of their COVID-19 status (Figure 1).5,20
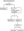
Figure 1: Flowchart for managing AD patients on oral and biologic agents during the COVID-19 outbreak
Because there is some risk with AD due to oral immunosuppression use, patients need to take every precaution, such as staying home, limiting interpersonal contact (avoiding groups or crowds of over 10 people), avoiding discretionary travel, practicing good hand hygiene, and wearing cloth face coverings in public settings where other social distancing measures are difficult to maintain.27,30
Conclusion
COVID-19 has been an international public health emergency that has infected more than 1,300,000 patients worldwide and impacted how health care providers of all specialties deliver care and administer treatment. In particular, the outbreak has affected how dermatologists care for patients with conditions, such as atopic dermatitis, and administer oral and biologic treatments. Dermatologists are tasked with balancing the risk of drug-related immunosuppression with the risk of urgent disease flares for patients continuing or discontinuing treatment.5 Nonetheless, AD patients should not discontinue oral and/or biologic treatment or delay treatment initiation unless they are >60 years old, infected with COVID-19, or have high-risk comorbidities.5,20 Patients should not stop oral and biologic therapy or other existing treatments without consulting their physicians.5 These recommendations may assist physicians in the management of AD patients on oral and biologic agents during the COVID-19 outbreak.
1. Guo Y-R, Cao Q-D, Hong Z-S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11-11.
2. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
3. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727-733.
4. Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nature Reviews Gastroenterology & Hepatology. 2020.
5. Guidance on treating patients with biologics. American Academy of Dermatology. https://assets.ctfassets.net/1ny4yoiyrqia/PicgNuD0IpYd9MSOwab47/07b614658aff5fc6ccc4c0bd910509a3/Biologics_and_COVID_19_FINAL_V2.pdf. Published 2020. Accessed2020.
6. Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4⁺ T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136-148.
7. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1-9.
8. Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020.
9. Rantala A, Jaakkola JJK, Jaakkola MS. Respiratory Infections in Adults with Atopic Disease and IgE Antibodies to Common Aeroallergens. PloS one. 2013;8(7):e68582.
10. Leung DYM. Infection in atopic dermatitis. Current Opinion in Pediatrics. 2003;15(4).
11. Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? The Journal of allergy and clinical immunology. 2014;134(2):247-259.
12. Ciprandi G, Tosca MA, Fasce L. Allergic children have more numerous and severe respiratory infections than non-allergic children. Pediatric Allergy and Immunology. 2006;17(5):389-391.
13. de Oliveira TB, Klering EA, da Veiga ABG. Is recurrent respiratory infection associated with allergic respiratory disease? Journal of Asthma. 2019;56(2):160-166.
14. Siegfried EC, Hebert AA. Diagnosis of Atopic Dermatitis: Mimics, Overlaps, and Complications. J Clin Med. 2015;4(5):884-917.
15. Eichenfield LF, Bieber T, Beck LA, et al. Infections in Dupilumab Clinical Trials in Atopic Dermatitis: A Comprehensive Pooled Analysis. American journal of clinical dermatology. 2019;20(3):443-456.
16. Ferrucci S, Romagnuolo M, Angileri L, Berti E, Tavecchio S. Safety of dupilumab in severe atopic dermatitis and infection of Covid-19: two case reports. Journal of the European Academy of Dermatology and Venereology. 2020;n/a(n/a).
17. Wollenberg A, Flohr C, Simon D, et al. European Task Force on Atopic Dermatitis (ETFAD) statement on severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2)-infection and atopic dermatitis. Journal of the European Academy of Dermatology and Venereology. 2020;n/a(n/a).
18. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) — United States FM, 2020. MMWR Morb Mortal Wkly Rep 2020;69:343-346. DOI: http://dx.doi.org/10.15585/mmwr.mm6912e2external icon. In.
19. McIntosh K. Coronavirus disease 2019 (COVID-19). 2020.
20. Shah P, Zampella JG. Use of Systemic Immunomodulatory Therapies During the Coronavirus Disease 2019 (COVID-19) Pandemic. Journal of the American Academy of Dermatology. 2020.
21. Wang C, Rademaker M, Baker C, Foley P. COVID-19 and the use of immunomodulatory and biologic agents for severe cutaneous disease: An Australia/New Zealand consensus statement. Australas J Dermatol. 2020.
22. Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10(3):330-335.
23. Salam N, Rane S, Das R, et al. T cell ageing: effects of age on development, survival & function. Indian J Med Res. 2013;138(5):595-608.
24. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. Journal of Travel Medicine. 2020;27(2).
25. Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072.
26. Pichard DC, Freeman AF, Cowen EW. Primary immunodeficiency update: Part I. Syndromes associated with eczematous dermatitis. Journal of the American Academy of Dermatology. 2015;73(3):355-366.
27. Association NE. Ask the Ecz-perts: Coronavirus (COVID-19). Ask the Ecz-perts Web site. https://nationaleczema.org/ate-covid-19/. Published 2020. Accessed2020.
28. Abramovits W. Atopic dermatitis. J Am Acad Dermatol. 2005;53(1 Suppl 1):S86-93.
29. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020.
30. Stop the spread. Centers for Disease Control and Prevention (CDC) Web site. https://www.cdc.gov/coronavirus/2019-ncov/communication/stop-the-spread.html. Published 2020. Accessed2020.
Ms. Uppal, Mr. Kearns, and Ms. Chat have no conflicts of interest to declare.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!





