Skin Bugs and Travel Bugs: An Update for Clinicians
When people travel on vacations, they tend to bring home souvenirs, photographs and memories. Sometimes, they also bring back travel-related diseases. Skin disease is the third most common medical problem that travelers encounter. In this article we will provide a brief overview on the clinical presentation and treatment for common travel-related skin diseases in current times.
Cutaneous larva migrans
Cutaneous larva migrans (CLM) is a very common travel-related skin disease most often acquired on beaches of Asia, Africa, South America, and the Caribbean. The condition can be identified with its characteristic serpiginous erythematous plaques and extreme pruritus. The hookworms Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala are usual intestinal parasites of dogs and cats. Eggs of the parasites are excreted in the host animal feces, and can invade humans through direct skin contact when walking barefoot in water or soil, typically on sandy beaches. These hookworms do not have the collagenase enzyme required to penetrate the basement membrane, hence they become trapped in the epidermis, resulting in the characteristic superficial snake-like appearance.

Cutaneous larva migrans. Cutaneous larva migrans. Serpiginous, erythematous linear plaques on abdomen
The skin infections are most commonly found on the feet, followed by the thighs and legs or buttocks. The lesions can remain dormant or can begin creeping activity at slow rate of 2–3cm/day, thus the term “creeping eruption.” Associated hypersensitivity reaction to the skin of the hookworm results in pruritus, peripheral eosinophilia, and elevated IgE. Potential complications include superinfection/impetiginization, bullae, papular urticarial, and rarely, Loeffler’s syndrome.
CLM is usually self-limiting and resolves after two to three months when the parasites die within the epidermis. However patients highly desire treatment. Anthelmintics such as ivermectin, albendazole or thiabendazole can be prescribed. Topical treatments such as cryotherapy are not usually effective.
Myiasis
Myiasis is infestation by larvae (maggots) of flies, commonly encountered in travelers returning from sub-Saharan Africa, Latin America, and the Caribbean. The larvae feed on the host’s dead or living tissue, body substances, or ingested food. Cutaneous presentations include follicular/furuncular myiasis, wound myiasis, and migratory/creeping myiasis, depending on the type of infesting larvae.

Myiasis. Furuncle with central punctum on the forearm.
Follicular/furuncular myiaisis occurs when larvae upon direct contact, burrow under the skin of the host, and feed on the host blood for five to 10 weeks. Once the larva matures, it emerges out of the skin, usually fall to the ground, and further mature into a fly in the soil. Follicular/furuncular myiasis is commonly caused by the human botfly Dermatobia hominis; the Tumbu fly Cordylobia anthropophaga; Cuterebra species, known as rabbit or rodent bot flies; and Wohlfahrtia vigil and W. opaca.
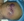
Furuncular Myiasis. Larvae extruding from furuncles.
Obasa TO, and Sowunmi FO. Myasis occuring in a neonate. Pediatr Rep. 2012 Dec 6; 4(4): e34. Under creative common license.

Myiasis. Furuncle with central punctum on the forearm. Used with permission from Dr. Bubna. Adapted from Bubna AK, Rangarajan S, Anandan S, Veeraraghavan M. Cicatricial alopecia as a sequel to furuncular myiasis of the scalp in an immunocompetent child. Indian J Paediatr Dermatol 2015;16:50-3
In some circumstances, when a fly infests open wounds, mucous membranes, such as nose and eye orbit, and body cavities, wound myiasis occurs. The female fly then lays eggs, that hatch after one to two days, and feed on tissue for approximately one week. As a result, the wound size increases and a characteristic odor is released that attracts more flies to lay eggs. Removal of larvae is difficult because spines on their body attach to the wound base. After maturing, the larvae fall to the ground and further mature into an adult fly. Wound myiasis is usually caused by the flies Cochliomyia hominivorax, Chrysomya bezziana, and Wohlfahrtia magnifica.
Lastly, in migratory/creeping myiasis, humans are accidental hosts. The female fly lays eggs on leg hairs of horses or cattle where they stay dormant. Humans are exposed to the eggs by contact with the horse’s coat or by accidental deposition of eggs by the fly onto human skin. On hatching, the larvae burrow into the epidermis and migrate one to 30cm/day; however, they ultimately die as they are unable to pupate. Migratory/creeping myiasis is commonly caused by the flies Gasterophilus intestinalis and Hypoderma ovis and lineatum.

Myiasis. Extracted larva.
Furuncular myiasis is treated by applying vaseline to the lesion for 24 hours, causing suffocation of the organism, and eventual emergence from the skin. A cruciform incision (not directly over the punctum) may also be done, allowing removal of the larvae. Migratory myiasis can be treated surgically with removal of the larva from the leading edge of the tract using a sterile needle or incision after anesthesia. Irrigation and debridement are also necessary for wound myiasis.
Tungiasis
Tungiasis, also called sand flea disease, is when the female sand flea Tunga penetrans burrows into the host skin after mating. Acral sites, especially toes and peri-ungual skin are the most common sites. It nourishes itself from the superficial dermal blood vessels, and extrudes more than 100 eggs, which subsequently fall to the ground. While implanted in the host skin, the flea grows approximately 2,000-fold in size in a time span of four to six weeks. It then dies and gets sloughed off from the host skin. Tungiasis is commonly found from the Caribbean, sub-Saharan Africa, India, and Pakistan. The sand flea can also be carried by animal hosts such as dogs, pigs, cows, and rats, which can lead to persistence of the organism in rural communities despite eradication efforts.
A papule or nodule with an overlying black dot where the flea has entered the host is the common presentation. Wearing adequate shoes is the best preventive measure against tungiasis. Treatment consists of physical removal of the organism, either through a simple shave or punch biopsy procedure, or with the use of a sterile needle. Topical ivermectin, metrifonate, or thiabendazole may also be used.
Leishmaniasis
Leishmaniasis is caused by the protozoan parasite of the genus Leishmania. The vector involved is the sand fly. It is usually classified into ‘Old World’ and ‘New World’ leishmaniasis. Old World (non-American) leishmaniasis comes from Africa, Asia, the Middle East, and Southern Europe (mostly from Iran, Afghanistan, Syria, and Saudi Arabia); New World (American) leishmaniasis can be found in Central and South America (usually Brazil and Peru), and sometimes as far north as Texas and Oklahoma.

Leishmaniasis. On the left a female sand fly is extracting blood. On the right oval and elongated leishmanial promastigotes. Note the anterior flagellum for motility.
Courtesy of D Evans. Wellcome Images
Old and New World leishmaniasis can be classified into four forms: localized cutaneous, diffuse cutaneous (or diffuse anergic cutaneous), mucocutaneous, and visceral. The progression from one stage to another largely depends on the Leishmania species and host response to the infection.
Cutaneous leishmaniasis presents as a papule that enlarges and forms larger nodules and plaques with indurated borders that may ulcerate. Diffuse (anergic) cutaneous leishmaniasis is a more extensive form of cutaneous leishmaniasis characterized by disseminated flesh-colored papules or nodules. Mucocutaneous leishmaniasis is a rare form that involves systemic presentations such as upper respiratory congestion and hoarseness, epistaxis with erythematous, edematous mucosa with purulent drainage. It can occur one to two years after the onset of primary cutaneous disease (progression rate of one to 10 percent). Severe cases can have mutilating destruction of the mucous membranes and surrounding cartilage, e.g. ulceration of the septal mucosae. Lastly, visceral/disseminated leishmaniasis, also known as Kala-azar (black sickness) is when there is systemic migration of infected tissue macrophages via the reticuloendothelial system. Presentations include fever, weight loss, weakness, pallor, hepatosplenomegaly, and lymphadenopathy. Ninety percent of visceral leishmaniasis originate from Bangladesh, Brazil, Ethiopia, India, Sudan, and South Sudan.
Pentavalent antimony (either intravenous sodium stibogluconate or intramuscular meglumine antimoniate) can be given for cutaneous leishmaniasis, depending on infecting species ( determined by PCR) and risk of progression to mucocutaneous disease. Patients with disseminated mucocutaneous leishmaniasis, visceral Old World leishmaniasis, and all patients with New World leishmaniasis should receive systemic antimony therapy. Side effects related include nausea, vomiting, diarrhea, fatigue, pancreatitis, cytopenias, and reversible EKG changes. Alternatively, oral miltefosine has been also been shown to be efficacious and well tolerated. In patients unresponsive to antimonials, liposomal amphotericin B may be used. Topical paromomycin, cryotherapy, or intra-lesional antimonials may also be used for uncomplicated localized disease in Old World leishmaniasis.
Chikungunya rash
Chikungunya fever is a viral disease contracted by humans through the bite of virus-carrying mosquitoes, A. albopictusand A. aegyptii. The disease usually originates from tropical Africa and Asia and is characterized by a sudden and severe fever, skin rash and joint and muscle pain. In Africa, wild primates such as monkeys and baboons are also involved in the transmission. Major outbreaks of chikungunya fever tend to occur cyclically, with a disease-free period of several years or decades between outbreaks.

Chikungunya. Diffuse hyperpigmentation with ill-defined borders in a neonate.
Courtesy of D Evans. Wellcome Images

Confluent vesicles and tense bullae on extremities.
Courtesy of Dr. Arun Inamadar
Chikungunya rash presents as a morbiliform eruption involving the upper limbs, trunk and face, typically asymptomatic or accompanied by mild pruritus. It appears within the first three to five days of the infection, and typically resolves in three to four days. As the rash resolves, characteristic hyperpigmentation appears, typically with centrofacial and freckle-like distribution, flagellate, and acneiform pigmentation in ultraviolet light exposed areas.
Ulcerations and vesico-bullous presentations can be present, particularly in infants. Initial skin manifestations may appear similar to those of dengue. The difference between chikungunya and dengue is that repeat infection of chikungunya will be milder than the first, while repeat dengue infection will be more severe. Aside from the fever and rash, there is associated severe myalgia and arthralgia, usually starting in the small joints of the hands and feet, wrists and ankles, then later affecting the larger joints. Other non-specific symptoms include headache, slight photophobia and insomnia.
Chikungunya is usually self-limiting. Antipyretics and nonsteroidal anti-inflammatory drugs (NSAIDs) may be given to control fever and joint pain. Fever usually disappears after two to three days. Muscle and joint pain usually lasts five to seven days, but in the elderly, it can persist for months. Sunscreens and topical corticosteroids should be considered to minimize skin hyperpigmentation. Ulcers should be cleaned and treated with topical antimicrobials when appropriate. Systemic steroid treatment may be required for more severe cutaneous lesions.
Jesitus J. UBM Medica. Chikungunya and Coxsackie Virus A6. http://dermatologytimes.modernmedicine.com/dermatology-times/news/chikungunya-and-coxsackievirus-a6-0. Published 2004. Accesses 15 May 2017.
Mesinkovska N. Travel maladies in the Americas: an overview. Lecture presented: 2016 American Academy of Dermatology Summer Meeting at Hynes Convention Center; July 28, 2016; Boston, MA.
Ngan V. New Zealand Dermatological Society. Chikungunya Fever. http://www.dermnetnz.org/topics/chikungunya-fever/. Published 2008. Accessed 15 May 2017.
Vasievich MP, Villarreal JDM, Tomecki KJ. Got the Travel Bug? A Review of Common Infections, Infestations, Bites, and Stings Among Returning Travelers. American Journal of Clinical Dermatology. 2016;17(5):451-462. doi:10.1007/s40257-016-0203-7.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!








