Systemic Inflammation in Psoriasis: A Guide for Dermatology Care Providers
Psoriasis is a chronic autoimmune disease affecting more than seven million adults in the United States, commonly presenting as epidermal plaques with skin erythema, induration, and adherent silvery-white scales.1 Psoriatic plaques can be pruritic, and visibility of the disease can cause embarrassment, shame, and stress, which can have a significant negative impact on emotional health and productivity.2
Psoriasis was originally considered a benign skin condition with minimal serious complications. However, numerous recent studies have unequivocally shown that psoriasis is a systemic inflammatory disease, and many of the key drivers of psoriasis are also implicated in the pathogenesis of other common chronic inflammatory diseases.3 Thus, patients with psoriasis are at increased risk for inflammatory comorbidities.4
This review discusses sources of systemic inflammation in psoriasis and how cytokines involved in psoriasis pathogenesis cause inflammation in other organ systems. Practical guidance is provided for dermatology care providers on the importance of screening patients with psoriasis for associated comorbidities.

Figure 1. Immunopathogenesis of Psoriasis5
Adapted from Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339-1350, with permission from Elsevier.
Abbreviations: DDC, dermal dendritic cells; IFN, interferon; IL, interleukin; KC, keratinocytes; KGF, keratinocyte growth factor; LC, lymphocytes; PDC, plasmacytoid dendritic cells; Tc, cytotoxic T cell; Th, T helper cell; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; VLA, very late antigen.
Systemic Inflammatory Burden of Psoriasis
Immunopathogenic pathways that stimulate inflammation and abnormal/excessive growth of skin cells in psoriasis are illustrated in Figure 1.5 Psoriasis can be triggered by environmental, infectious, and genetic factors, which place stress on keratinocytes. These triggers initiate a cascade of events, including activation of dendritic cells and differentiation of naïve T cells into T helper 1 and T helper 17 cells, which mediate immune responses characterized by release of pro-inflammatory cytokines. Key cytokines in these pathways include tumor necrosis factor (TNF)-α, interleukin (IL)-17, IL-22, IL-23, IL-6, IL-1β, and interferon-γ.6
In uncontrolled psoriasis, levels of inflammatory cytokines are increased in skin lesions and blood plasma.7 Such a systemic increase results in chronic inflammation throughout the body, including in the heart, liver, kidneys, intestines, muscles, and tendons.3,8,9 Systemic inflammation is present most frequently in moderate-to-severe psoriasis; however, recent studies have shown that subclinical vascular and hepatic inflammation is also present in mild psoriasis.10,11
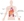
Figure 2. Comorbidities Associated With Psoriasis
This systemic inflammation, driven by a common set of cytokines, increases risk for comorbidities, including cardiovascular disease, psoriatic arthritis, chronic kidney disease, metabolic syndrome, and non-alcoholic fatty liver disease (Figure 2).4,12,13 For example, patients with psoriasis are at significantly higher risk than individuals without psoriasis for comorbid cardiovascular disease, and comorbid metabolic syndrome and each of its components (Table 1).14-18 Furthermore, psoriasis is associated with an estimated 10-year increased risk for major adverse cardiac events of 6.2 percent, including significantly higher risk for death compared with the general population.19 Imaging studies suggest that increased cardiovascular risks in psoriasis may be associated with increased aortic wall inflammation, subcutaneous adipose tissue inflammation, and atherosclerosis.20,21

The systemic increase in pro-inflammatory cytokine levels (eg, TNF-α, IL-6, and IL-17) promotes insulin resistance and metabolic abnormalities, which increase the risk for obesity, diabetes mellitus, and non-alcoholic fatty liver disease.22-24 Increased plasma levels of these cytokines cause increased renal inflammation and immune-mediated kidney damage in psoriasis, providing a possible explanation for a 1.9-fold to 3-fold increased risk for kidney disease and up to 4-fold increased risk for death from kidney disease in severe psoriasis.12,25
Another common comorbidity, affecting 30 percent of patients with psoriasis, is psoriatic arthritis, a form of inflammatory arthritis that commonly presents with asymmetrical joint pain, swelling, and stiffness.26 Psoriatic arthritis pathogenesis is linked with upregulation of IL-17, IL-23, IL-8, and other pro-inflammatory chemokines and cytokines.27
Screening Patients With Psoriasis for Associated Comorbidities
In the field of dermatology, guidance is lacking regarding how and when to screen patients with psoriasis for common cardiometabolic comorbidities. In the authors’ opinions, dermatologists, nurse practitioners, and physician assistants can play an important role in ensuring that such screenings are performed, along with well-established screenings (eg, tuberculosis prior to biologic initiation and monitoring for infections or malignancies). Comorbidity screenings for patients with psoriasis should include taking a complete medical history, thorough physical examination, blood pressure measurement, and a comprehensive metabolic panel, including liver and kidney function, and measurements of glucose, triglyceride, and cholesterol levels (Table 2). Patients meeting three or more of the criteria from Table 3 have metabolic syndrome.


Dermatology care providers knowledgeable about psoriasis and associated comorbidities can provide proper referral to a primary care physician or specialist. Interdisciplinary collaboration between dermatologists, primary care, and other specialists is important to ensure that care is coordinated and patients receive appropriate follow-up for optimal management of comorbidities
Dermatology care providers can also help by encouraging patients to make healthy lifestyle choices, including following a healthy diet, obtaining adequate physical activity, and avoiding smoking and excessive alcohol consumption. Patients should also be advised to see their primary care physicians for screenings or preventive services that are not part of routine dermatologic care. Additionally, by providing patient education related to psoriasis being a systemic disease, dermatology care providers can encourage patients to become more involved in their disease management and self-care.
Treatment of Psoriasis as a Systemic Disease
Choice of psoriasis treatment is generally based on severity of skin disease and impact on quality of life, although clinicians should also consider the effects of different treatments on systemic inflammation and associated comorbidities. Of particular concern for patients with moderate-to-severe psoriasis is the observation that comorbid cardiovascular disease shortens the average lifespan by five to six years compared with patients with mild psoriasis.25,28 However, it is not yet known if reducing psoriasis severity can lengthen patient lifespan. Table 4 summarizes a population-based study evaluating differences in risks for associated comorbidities by psoriasis severity.29 Risk for many inflammatory comorbidities increases significantly with increasing psoriasis severity.


Because topical therapies and ultraviolet B phototherapy are applied locally to psoriatic lesions, these treatments may primarily have local effects. In contrast, some conventional oral systemic therapies, especially methotrexate, are associated with reductions in cardiovascular risk in patients with psoriasis and inflammatory arthritis.30,31 Possible effects of methotrexate on inflammation are being tested in an ongoing study (the Cardiovascular Inflammation Reduction Trial [CIRT]; NCT01594333), evaluating the effects of low-dose methotrexate on major adverse cardiac events incidence in approximately 7,000 patients with type 2 diabetes or metabolic syndrome with history of coronary artery disease.32
While conventional systemic treatments may improve skin symptoms and methotrexate may reduce cardiovascular risk, these therapies are associated with systemic side effects, which can negatively impact other organ systems and increase risks for comorbidities (Table 5).33,34
Since 2003, eight new biologics have been approved for treatment of moderate-to-severe psoriasis. These therapies target key inflammatory cytokines (ie, TNF-α, IL-17A, IL-12/23, and IL-23) associated with psoriasis pathogenesis. The newest biologics have minimal screening requirements and no requirements for ongoing safety monitoring. Thus, in our opinion, providers must be diligent in screening for comorbidities associated with psoriasis and not become complacent in screening for comorbidities due to the safety profiles of newer biologics. To holistically care for and manage patients with moderate-to-severe psoriasis, providers must fully recognize the systemic nature of the disease.
Retrospective studies have shown that TNF-α inhibitors (eg, adalimumab, etanercept, and infliximab) are associated with cardiometabolic benefits in psoriasis, including reduced incidence of myocardial infarction, improved insulin sensitivity, and prevention of progression of liver injury.35,36 However, a recent randomized, placebo-controlled trial of adalimumab in psoriasis showed that after 16 weeks of treatment, vascular inflammation levels as measured by 18fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography were not statistically different with TNF-α therapy compared with placebo.37 The ongoing Vascular Inflammation in Psoriasis (VIP) study of adalimumab (NCT01553058) and the VIP extension study (NCT01866592) will provide further insight into the effects of TNF-α inhibition on vascular inflammation, lipid metabolism, and inflammatory biomarker levels.
The effects of IL-12/23 inhibition on inflammation and risk for cardiovascular events are poorly understood.28 In a study of 10 patients with moderate-to-severe psoriasis receiving ustekinumab who achieved 75 percent improvement in baseline Psoriasis Area and Severity Index, inflammation of the liver, spleen, and aorta as measured by 18fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography were significantly decreased compared with age, gender, and body mass index-matched controls.38 The ongoing VIP-Ustekinumab (VIP-U; NCT02187172) study will provide additional data from patients with psoriasis on the effects of IL-12/23 inhibition on cardiometabolic risk.
Because IL-17 promotes vascular inflammation, endothelial dysfunction, and arterial hypertension in experimental models of psoriasis,39,40 it is hypothesized that monoclonal antibodies neutralizing IL-17A (eg, secukinumab and ixekizumab) could reduce inflammation associated with liver and kidney diseases, obesity, hypertension, and atherosclerosis. The ongoing VIP-Secukinumab (VIP-S; NCT02690701) study will evaluate the effects of secukinumab treatment compared with placebo on aortic vascular inflammation in poorly controlled moderate-to-severe plaque psoriasis. Additionally, another study of secukinumab (NCT03055494) is underway, which will evaluate the effect of secukinumab treatment compared with placebo on adipose tissue in moderate-to-severe plaque psoriasis.
Conclusion
Psoriasis is a systemic, inflammatory disease with associated comorbidities, in which the role of important cytokines, such as TNF-α, IL-17, and IL-23, have not been well understood until recently. Dermatology care providers need to consider this association when screening patients for psoriasis and when determining optimal strategies to maximize treatment benefits while balancing known risks. Until the spectrum of disease severity and inflammation is better understood, clinicians should focus their efforts on being alert and sensitive to the myriad inflammatory effects known to occur in patients with psoriasis and to consider these issues as part of the appropriate treatment of patients with psoriasis.
Acknowledgements
Technical assistance with editing and styling of the manuscript for submission was provided by Oxford PharmaGenesis Inc. and was funded by Novartis Pharmaceuticals Corporation. The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this manuscript.
Conflicts of Interest
Wendy Cantrell served as an investigator for Eli Lilly, Pfizer, Novartis, Janssen, Merck, AbbVie, and Amgen; and a consultant for Eli Lilly, Pfizer, and Novartis.
Joe Gorelick served as a speaker for AbbVie, Allergan, Aqua, Eli Lilly, Novartis, Galderma, Bayer, Cipher, Dusa, Leo, Medimetriks, Promius, PuraCap, Ranbaxy, and Taro; a consultant for Allergan; and an advisory board member for Allergan, Novartis, Galderma, Celgene, Exceltis, Leo, and Regeneron.
Kristine Kucera served as a speaker or adviser for AbbVie, Bayer, Janssen, Novartis, Promius, Celgene, Encore, Anacor, GenRX, Regeneron, and Valeant; and a consultant for Eli Lilly.
Scott Freeman served as an investigator for DUSA; a speaker for Galderma, Leo, Bayer, Aqua, Promius, and GenRx; and an advisory board member for LeoPharma, Celgene, Novartis, and Genentech.
1. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512-516.
2. Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20(8):pii: http://www.escholarship.org/uc/item/48r44w48h42.
3. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130(7):1785-1796.
4. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol. 2017;76(3):377-390.
5. Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339-1350.
6. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496-509.
7. Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005(5):273-279.
8. Grozdev I, Korman N, Tsankov N. Psoriasis as a systemic disease. Clin Dermatol. 2014;32(3):343-350.
9. Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147(9):1031-1039.
10. Dave J, Ahlman MA, Lockshin BN, Bluemke DA, Mehta NN. Vascular inflammation in psoriasis localizes to the arterial wall using a novel imaging technique. J Am Acad Dermatol. 2014;70(6):1137-1138.
11. Youn SW, et al. Subclinical systemic and vascular inflammation detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with mild psoriasis. J Dermatol. 2015;42(6):559-566.
12. Chi CC, Wang J, Chen YF, Wang SH, Chen FL, Tung TH. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: A nationwide population-based cohort study. J Dermatol Sci. 2015;78(3):232-238.
13. Girolomoni G, Gisondi P. Psoriasis and systemic inflammation: underdiagnosed enthesopathy. J Eur Acad Dermatol Venereol. 2009;23 Suppl 1:3-8.
14. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68(4):654-662.
15. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84-91.
16. Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31(3):433-443.
17. Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2(2):e000062.
18. Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168(3):486-495.
19. Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124(8):775.e1-6.
20. Hjuler KF, Gormsen LC, Vendelbo MH, Egeberg A, Nielsen J, Iversen L. Increased global arterial and subcutaneous adipose tissue inflammation in patients with moderate-to-severe psoriasis. Br J Dermatol. 2017;176(3):732-740.
21. Santilli S, Kast DR, Grozdev I, et al. Visualization of atherosclerosis as detected by coronary artery calcium and carotid intima-media thickness reveals significant atherosclerosis in a cross-sectional study of psoriasis patients in a tertiary care center. J Transl Med. 2016;14(1):217.
22. Candia R, Ruiz A, Torres-Robles R, Chávez-Tapia N, Méndez-Sánchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29(4):656-662.
23. Fleming P, Kraft J, Gulliver WP, Lynde C. The relationship of obesity with the severity of psoriasis: a systematic review. J Cutan Med Surg. 2015;19(5):450-456.
24. Reich K. The concept of psoriasis as a systemic inflammation: implications for disease management. J Eur Acad Dermatol Venereol. 2012;26 Suppl 2:3-11.
25. Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163(3):586-592.
26. Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729-735.
27. Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13(4-5):496-502.
28. Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33(1):41-55.
29. Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173-1179.
30. Boehncke S, Salgo R, Garbaraviciene J, et al. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol. 2011;25(10):1187-1193.
31. Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262-267.
32. Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(2):199-207.e15.
33. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: Implications for management. J Am Acad Dermatol. 2017;76(3):393-403.
34. Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29(3):157-178.
35. Wu JJ, Poon KY. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13(8):932-934.
36. Campanati A, Ganzetti G, Di Sario A, et al. The effect of etanercept on hepatic fibrosis risk in patients with non-alcoholic fatty liver disease, metabolic syndrome, and psoriasis. J Gastroenterol. 2013;48(7):839-846.
37. Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137(8):1638-1645.
38. Lee W, Kim B. Effects of ustekinumab on systemic and vascular inflammation assessed by 18F-FDG PET/CT in Korean patients with moderate to severe psoriasis [abstract]. J Am Acad Dermatol. 2017;76(6 Suppl 1):AB110. Abstract 5608.
39. Karbach S, Croxford AL, Oelze M, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol. 2014;34(12):2658-2668.
40. Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55(2):500-507.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!









