A Complicated Case of Recalcitrant Lichen Planus with Underlying Squamous Cell Carcinoma Treated with 5-Fluorouracil
Lichen planus is a chronic inflammatory condition that affects the skin and mucosal surfaces. The classic presentation of cutaneous lichen planus is often characterized using the six Ps: planar, purple, polygonal, pruritic, papules, and plaques. However, there are several clinical subtype,s each with their own unique morphology and associated histopathology.1 The hypertrophic variant commonly presents with scaly hyperkeratotic, elevated plaques demonstrating follicular accentuation, symmetrically distributed along the ankles, anterior surface of the lower extremities, and interphalangeal joints, and ranging in color from red-brown to purple-gray.2,3 Histopathology of hypertrophic lichen planus classically demonstrates hyperkeratosis, hypergranulosis, and acanthosis with rounded and elongated rete ridges, rather than the characteristic sawtooth pattern seen in typical lichen planus.2
Although the exact etiology remains unclear, the most widely accepted theory is that lichen planus is an autoimmune disease in which cytotoxic T cells damage basal keratinocytes at the dermal-epidermal junction.1 Other etiologies include, but are not limited to, genetic factors, dental materials, infectious agents, chronic inflammatory states, drugs, and malignancy.1 Various materials used in routine dental procedures, including silver amalgam, gold, chromium, and prolonged use of dentures, have been shown to trigger oral lichen planus eruptions.4 Chronic inflammatory states such as ulcerative colitis, Crohn’s disease, and chronic hepatitis C infection, as well as infections with various bacterial agents, have also been shown to be associated with this condition.1 The association between lichen planus and malignancy has been well-documented in the literature.5 The phenomenon of keratoacanthomas arising in hypertrophic lichen planus was first documented in 1981, and since then, several cases have been reported of squamous cell carcinoma arising in hypertrophic lichen planus.5-8
Several drug-induced lichenoid reactions have been described in the literature as well. They can occur as a side effect of medications including angiotensin-converting enzyme inhibitors, thiazide diuretics, beta blockers, anti-malaria drugs, and chemotherapeutic agents.1 One chemotherapeutic agent is 5-fluorouracil (5-FU), which inhibits the enzyme thymidylate synthase, preventing the addition of fluoronucleotides into the DNA sequence, and thus preventing DNA replication.9 5-FU can be used to treat a wide range of cancers, including colorectal, breast, and non-melanoma skin cancers.9 Capecitabine, an oral pro-drug of 5-FU, has a well-documented history of causing drug-induced lichen planus eruptions, but such reactions secondary to topical or intralesional 5-FU have not been described in current literature.10
Case Report
Our patient is an 80-year-old Caucasian woman with a recalcitrant complicated case of hypertrophic lichen planus. She originally presented to the dermatology clinic for Mohs surgery of a single well-differentiated squamous cell carcinoma of the right anterior lower leg, which was further complicated with new-onset lichen planus during her squamous cell carcinoma treatment (Figure 1). This patient also had a pertinent past medical history of a chronic tooth abscess and numerous amalgam tooth fillings, which were both still present throughout the course of this case.

Figure 1. Patient’s initial presentation with erythematous hyperkeratotic papules and plaques of the pretibial regions bilaterally concerning for actinic keratosis and squamous cell carcinoma.
Following excision of her squamous cell carcinoma, the patient was noted to have numerous suspicious lesions suggestive of actinic keratosis and squamous cell carcinoma on her lower legs bilaterally. Excisions were avoided and topical treatment was pursued given poor wound healing on her recent Mohs surgery and presence of stasis dermatitis. The patient was ultimately treated for 5 months with curettage, followed by intralesional injections of 5-FU into four keratotic nodules highly suspicious for squamous cell carcinoma located on her lower extremities. In addition, she was also concurrently treated with 5-FU cream under Unna wraps.
Two months into treatment with both topical and intralesional 5-FU, the patient experienced worsening erythematous, irritated, pruritic lesions on her lower legs bilaterally. Upon examination of the pretibial and lateral regions of the lower extremities, she was noted to have diffuse erythema and edema with associated satellite pustules and yellow, hyperkeratotic plaques with slight scale (Figure 2). Lesions were localized to the lower extremities bilaterally, and the patient denied any systemic symptoms such as fever or chills. Bacterial and fungal cultures were collected and the patient was started on empiric treatment with 100 milligrams of fluconazole daily for seven days, 100mg of doxycycline two times daily for 14 days, and diluted bleach baths.

Figure 2. Bilateral diffuse erythematous pink papules and plaques with associated satellite pustules and yellow, hyperkeratotic plaques with slight scale following two months of treatment with intralesional 5-FU and topical 5-FU under Unna wraps.
Follow-up one week later showed only mild improvement of bilateral lower extremity lesions. Both the preliminary fungal and bacterial cultures showed no abnormal growth. Infectious disease was consulted and fluconazole was increased to 200mg daily for one week. The patient also continued diluted bleach and vinegar soaks but despite this, her symptoms persisted with no significant change. Due to the refractory nature of the skin eruption, several shave biopsies were performed (Figure 3). Histopathology demonstrated epidermal acanthosis, wedge-shaped hypergranulosis, a sawtooth rete ridge pattern with necrosis of the basal layer, and a band-like lymphoid infiltrate, findings consistent with a diagnosis of hypertrophic lichen planus (Figure 4). Subsequent hepatitis C testing was performed and results were negative.
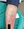
Figure 3. Erythematous tender nodule with hyperkeratotic scale located on the left proximal pretibial region. Biopsy by shave method was performed at marked site with histopathologic findings demonstrated in Figure 4.

Figure 4. Histopathologic section demonstrating epidermal acanthosis, wedge-shaped hypergranulosis, a sawtooth rete ridge pattern with necrosis of the basal layer, and a band-like lymphoid infiltrate, findings consistent with a diagnosis of hypertrophic lichen planus (Hematoxylin-eosin stain; original magnification: x10).
After receiving a diagnosis of lichen planus, the patient was treated with various combinations of topical steroids, vitamin A derivatives, and bleach baths over several months, but showed little improvement. At this time, the patient was still undergoing concurrent additional rounds of intradermal injections of 5-FU for lesions suspicious of squamous cell carcinoma in the pretibial regions bilaterally. Treatment with intralesional 5-FU was discontinued for a trial period. The patient’s lichen planus only began to improve once 5-FU treatments were discontinued, in combination with continued application of topical clobetasol 0.05% and tazarotene cream (Figure 5).

Figure 5. Two months after the removal of 5-FU treatment and concurrent treatment with clobetasol and tazarotene.
Although the patient’s cutaneous reaction has not completely cleared, it has significantly improved with combination treatment of tazarotene and clobetasol along with discontinuation of the concurrent 5-FU treatment for squamous cell carcinoma. Most recently, the patient’s oral abscess was drained and the infected tooth was extracted. She plans to follow-up to evaluate if her lichen planus reaction has further improved. If her lesions fail to clear, she will consider replacing her amalgam fillings.
Discussion
The 80-year-old female in this report has a complicated case of lichen planus with four possible etiologies: transient bacteremia from a chronic tooth abscess; amalgam fillings in her teeth leading to lichen planus; topical and intralesional 5-FU treatments for squamous cell carcinoma; pre-existing lichen planus worsened by 5-FU treatment. Our patient’s lichen planus presented as yellow hyperkeratotic plaques with slight scale and diffuse erythema and edema on her anterior shins bilaterally upon initiation of treatment for her presumed squamous cell carcinoma. Because severe cases of hypertrophic lichen planus have been documented to mimic well-differentiated squamous cell carcinoma, clinical diagnosis and differentiation between the two conditions can be difficult. Our patient was empirically started on topical and intralesional 5-FU after presenting with hyperkeratotic plaques and signs of actinic damage concerning for pre-cancerous and cancerous lesions. However, due to poor wound healing in prior excisions, biopsies and histopathologic confirmation of these initial lesions was not performed. As a result, it can not be concluded with certainty whether this patient had cutaneous malignancy or early evolving hypertrophic lichen planus at initial presentation. Initiating topical chemotherapeutic agents for what was initially presumed to be lesions of actinic keratosis and squamous cell carcinoma in our patient ultimately led to a worsened presentation of lichen planus. Regardless of etiology, these lesions only showed improvement with cessation of topical and intralesional 5-FU treatments and concurrent topical tazarotene and clobetasol application. We propose that delivery of topical and intralesional 5-FU may have an additive effect on lichen planus, especially given the temporal and spatial correlation in our patient.
Conclusion
This novel case report details improvement of lichen planus on the lower extremities upon cessation of 5-FU. Due to the similar clinical presentation of squamous cell carcinoma, early clinical identification of this reaction may be delayed, and thus further investigation and awareness of this association needs to be explored. This case report contributes to the growing research on drug interactions and lichen planus and aids in the clinical diagnosis of potential dermatologic side effects from medications such as topical and intralesional 5-FU. In similar cases of patients with lichen planus, early review of medications may be indicated to prevent potential complications associated with 5-FU treatment. Additionally, this report emphasizes the importance of clinicopathologic correlation, as hypertrophic lichen planus and squamous cell carcinoma share several clinical and histopathologic features.
No funding was provided for the purpose of this report. The authors have no conflicts of interest to disclose.
Le CL, Olivier C. Lichen planus. N Engl J Med. 2012;366(8):723-732.
Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826.
Welsh J, Skvarka C, Allen H. A Novel Visual Clue for the Diagnosis of Hypertrophic Lichen Planus. Arch Dermatol. 2006;142(7):954.
Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian Journal of Dermatology. 2015;60(3):222-229.
Sigurgeirsson B, Lindelöf B. Lichen Planus and Malignancy: An Epidemiologic Study of 2071 Patients and a Review of the Literature. Arch Dermatol. 1991;127(11):1684–1688.
Allen JV, Callen JP. Keratoacanthomas Arising in Hypertrophic Lichen Planus: A Case Report. Arch Dermatol.1981;117(8):519–521.
Haenen CCP, Buurma AAJ, Genders RE, et al. Squamous cell carcinoma arising in hypertrophic lichen planus. BMJ Case Reports. 2018;bcr-2017-224044.
Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: The importance of clinicopathologic correlation. JAAD Case Rep. 2017;3(2):151-154.
Longley, D. B., Harkin, D. P., & Johnston, P. G. (2003). 5-Fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer, 3(5), 330+.
Walker G, Lane N, Parekh P. Photosensitive lichenoid drug eruption to capecitabine. Journal of the American Academy of Dermatology. 2014;71(2):52-53.
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!





