Patients diagnosed with stage III melanoma have historically faced a poor long-term prognosis. With successful surgical resection and interferon-alpha therapy (IFN-a), only about 45 percent of patients are disease-free at four years, and data suggest the benefits of INF-a may be limited in those with macroscopic involvement or non-ulcerated lesions.1 However, the latest data for the cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody ipilimumab (Yervoy, Bristol-Myers Squibb), show that it may confer benefits in terms of disease-free and overall survival in patients with resected stage III melanomas, regardless of whether subjects had micro- or macroscopic involvement or ulceration. Importantly, the 10mg/kg dosing that was studied in the recently reported European Organization for Research and Treatment of Cancer (EORTC) 18071 trial is higher than that used in initial studies, and it was associated with a substantial risk of toxicity. Nonetheless, the findings offer hope to those diagnosed with advanced stage melanoma and indicate a need to be better able to discriminate melanoma stage at diagnosis.
Ipilimumab Data
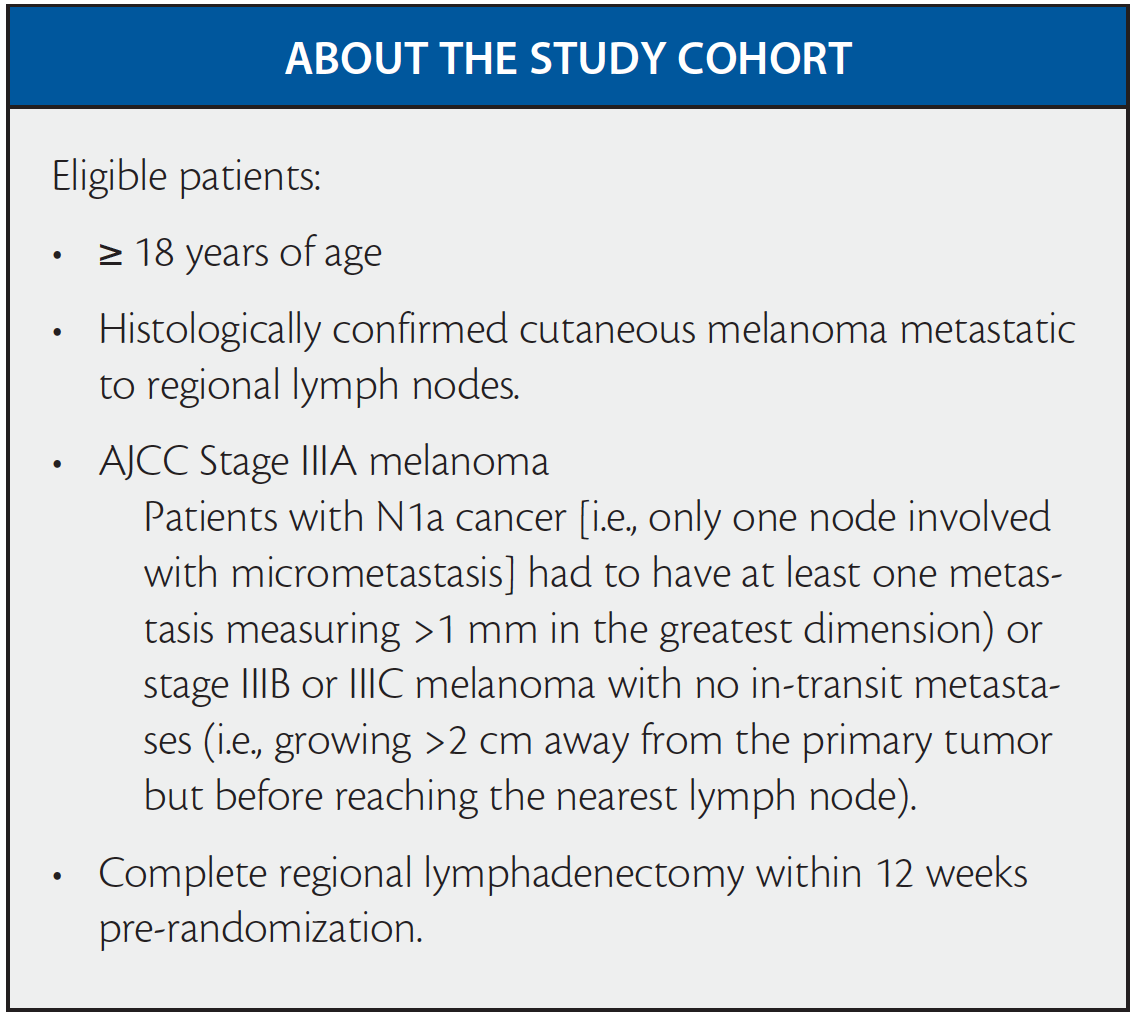
Ipiliumumab was FDA approved in 2011 at a dose of 3mg/kg for the treatment of advanced melanoma. Based on promising phase 2 trial data, researchers undertook the phase III EORTC 18071 trial to assess outcomes with a 10mg/kg dose in patients who had resected regional lymph node–positive (stage III) melanoma with a high risk of recurrence. The randomized, double-blind, phase 3 trial enrolled patients at 99 centers in 19 countries. The findings—with overall median follow-up of 5.3 years—expand the picture from the publication of interim findings that had 2.7 years follow up.
Patients were randomly assigned in a 1:1 ratio to receive an IV infusion of ipilimumab at a dose of 10mg/kg or placebo every three weeks for four doses, then every three months for up to three years. Causes for discontinuation included disease recurrence, an unacceptable level of toxic effects, a major protocol violation, or withdrawal of consent.
The primary end point was recurrence-free survival. Secondary end points included overall survival, distant metastasis–free survival, safety, and health-related quality of life.
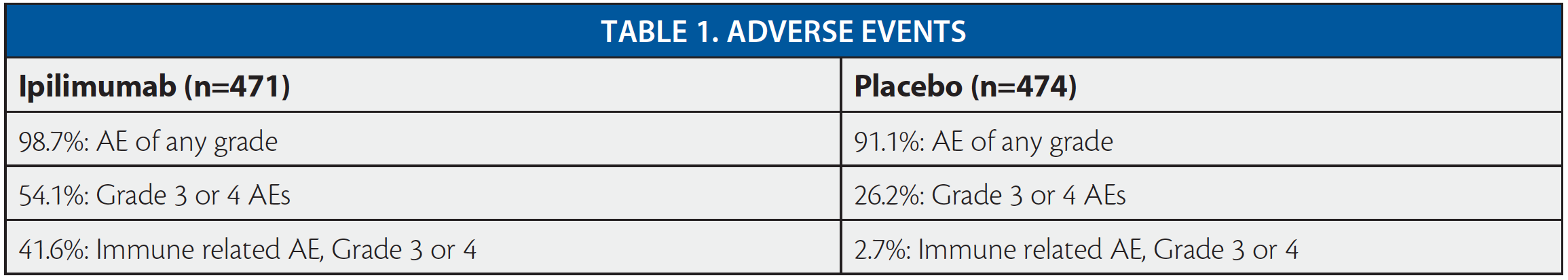
A total of 951 patients underwent randomization: 475 patients were assigned to the ipilimumab group and 476 to the placebo group.
The recurrence-free survival rate at five years was 40.8 percent in the ipilimumab group, compared with 30.3 percent in the placebo group The authors note that while the study was not powered to allow subgroup analysis, they observed that the prolongation of recurrence-free survival attributable to adjuvant ipilimumab appeared to be consistent across subgroups; specifically, survival benefit appeared to be consisted in those with both microscopic and macroscopic involvement.
The overall survival rate at five years was 65.4 percent in the ipilimumab group, versus 54.4 percent in the placebo group. Overall survival and metastasis survival was significantly longer in the ipilimumab group than in the placebo group.
Roughly half (53.3 percent) of subjects who started ipilimumab discontinued treatment due to an adverse event. Among these, investigators determined about half (51 percent) of those events were drug-related. In contrast, only 4.6 percent of 474 patients who received placebo discontinued treatment due to an adverse event.
Investigators determined that treatment with ipilimumab 10mg/kg reduced the risk of the risk of distant metastasis or death by 24 percent. At five years, ipilimumab provided approximately 10 percent higher rates of recurrence-free survival (40.8 vs. 30.3 percent), overall survival (65.4 vs. 54.4 percent), and distant metastasis–free survival (48.3 vs. 38.9 percent), compared to placebo.
Implications
Sentinel lymph node biopsy has been identified as an important tool for melanoma staging when coupled with other factors, such as Breslow thickness. Macroscopic involvement of the nodes is generally a poor prognostic indicator, and previously available therapies, such as IFN-a, were not generally beneficial for such cases. Ipilimumab may represent a treatment option for these patients.
Importantly, newer tools have emerged, such as gene expression profiling (GEP), to assist in melanoma staging. GEP (DecisionDx, Castle Biosciences) uses reverse transcription polymerase chain reaction (RT-PCR) to assess the expression of 31 genes from primary melanoma tumors; findings are considered along with SLNB outcome for each patient. GEP outcome was shown to be a more significant and better predictor of disease-free, distant metastasis-free, and overall survival in univariate and multivariate regression analysis, compared with SLNB.2
Armed with a more accurate staging and improved knowledge of the individual’s risk for developing metastasis or recurrence, dermatologists can better engage in a discussion about the potential benefits of a treatment like ipilimumab, relative to the risks. For individuals with stage III melanoma who can tolerate ipilimumab treatment, there could be a benefit.

Jonathan Wolfe, MD is an Associate Professor of Dermatology at the University of Pennsylvania.
1. Eggermont AM, Suciu S, Rutkowski P, et al. Long term follow up of the EORTC 18952 trial of adjuvant therapy in resected stage IIB-III cutaneous melanoma patients comparing intermediate doses of interferon-alpha-2b (IFN) with observation: ulceration of primary is key determinant for IFN-sensitivity. Eur J Cancer2016;55:111-121
2. Gerami P, Cook RW, Russell MC, Wilkinson J, Amaria RN, Gonzalez R, Lyle S, Jackson GL, Greisinger AJ, Johnson CE, Oelschlager KM, Stone JF, Maetzold DJ, Ferris LK, Wayne JD, Cooper C, Obregon R, Delman KA, Lawson D. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J Am Acad Dermatol. 2015 May;72(5):780-5.
Recommended
- Skin Cancer & Photoprotection
DermWireTV Extra: Dr. Bhatia and Dr. Schlesinger Discuss Collaboration With Oncologists










